Interpretable machine learning for predicting optimal surgical timing in polytrauma patients with TBI and fractures to reduce postoperative infection risk
作者:Sang, Xi-Guang
Introduction
Polytrauma, involving multiple bodily systems and organs, is a severe form of injury that frequently includes traumatic brain injury (TBI) and fractures1. Patients with concomitant traumatic brain injury and fractures require an integrated treatment approach to optimize their prognosis2,3,4,5. Postoperative infection is a common complication of surgery, with severe cases potentially leading to patient mortality or long-term disability6,7,8. The appropriate timing of surgery is crucial for several reasons: it can reduce the complexity of fracture reconstruction, minimize or prevent surgical site infections, and is instrumental in the recovery of limb function and the rehabilitation of brain injuries9,10,11,12. Current research suggests that fracture care should not be delayed unnecessarily, and the significance of physiological response to surgical decision-making has become more evident13,14,15. Fracture repair surgery should be carried out as soon as possible after adequate resuscitation and stabilization. Therefore, determining the optimal timing for fracture reconstruction surgery in patients with traumatic brain injury and fractures is essential for reducing the risk of postoperative infection and improving patient outcomes.
Despite clinical guidelines and experience-based decision-making, these processes often lack personalization and do not fully consider the specific circumstances of each patient16,17,18. With the rapid development of machine learning technology, its application in the medical field has become increasingly widespread, particularly in supporting complex clinical decisions. Machine learning algorithms can process vast amounts of clinical data, identify key factors affecting surgical conditions and timing and patient prognosis, and provide more precise and personalized recommendations for clinical decision-making19,20,21.
This study aims to utilize machine learning technology to initially explore the optimal conditions and timing for fracture reconstruction surgery in patients with traumatic brain injury and fractures due to polytrauma, in order to reduce the risk of postoperative infection. We designed seven different machine learning algorithms to analyze and predict various independent variables affecting postoperative infection, including patients’ baseline characteristics, types of injuries, and surgical features. Through this study, we aim to provide clinicians with a useful tool to help them better determine the timing of surgery, potentially improving patient treatment outcomes. Additionally, this study may offer new perspectives and methods for future clinical practice and research.
Methods
Study population
The study population consisted of polytrauma patients admitted to the Emergency Surgery Department of Qilu Hospital, Shandong University, from July 2011 to April 2024, including those with traumatic brain injury and surgically treated fractures, totaling 218 patients. Inclusion criteria were as follows: 1. Diagnosed with traumatic brain injury, combined with limb and pelvic fractures requiring surgery, 2. Initial care and fracture reconstruction surgery were conducted at our institution, 3. Age equal to or greater than 16 years old. Exclusion criteria included: 1. Patients with a history of significant trauma leading to disability, 2. Concurrent severe underlying diseases making them unable to tolerate surgery, 3. Interruption of the diagnostic and treatment process, including patients discharged without receiving fracture reconstruction surgery and died in the emergency department or while hospitalized without having the surgery, 4. Patients with pathological fractures, 5. Incomplete medical record documentation, including incomplete patient information and missing surgical records. Patients were categorized into the Infection group and the Non-Infection group based on the occurrence of postoperative nosocomial infection events.
Ethical clearance was granted for this research by the Research Ethics Committee of Qilu Hospital, Shandong University, which waived the requirement for informed consent due to the study’s retrospective design. Adherence to the Declaration of Helsinki’s ethical guidelines was maintained throughout the research process. The Research Ethics Committee of Qilu Hospital, Shandong University, approved the study (KYLL-202405-044).
Predictive variables
The collected patient data encompassed demographic characteristics, physiological status at admission, and non-invasive test indicators at admission and the day prior to surgery. Demographic features included age, sex, smoking and alcohol consumption status. Variables related to the admission status comprised heart rate, respiratory rate, blood pressure, shock index (pulse rate divided by systolic blood pressure), level of consciousness as measured by the Glasgow Coma Scale (GCS score ranging from 3 to 15), and fracture site. Non-invasive test indicators included D-dimer levels, prothrombin time-international normalized ratio (PT-INR), hematocrit (hct), and lactate levels (lac) upon admission, as well as low-density lipoprotein cholesterol (LDL-C), serum calcium (Ca2+), percentage of neutrophils (neu%), lymphocyte count (lym), hemoglobin (hgb), and platelet count (plt) one day before surgery. In our treatment process, we have incorporated to some extent the philosophies of"Damage Control Orthopedics (DCO)“and”Safe Definitive Orthopedic Surgery (SDS)". Consequently, we have included the number of surgical interventions prior to the repair of“major fractures”, encompassing all procedures conducted in the operating room13. Intraoperative or postoperative variables were not assessed as candidate input variables, emphasizing the development of a preoperative predictive model to aid in therapeutic decision-making.
Outcomes
The primary outcome of this study was the incidence of postoperative nosocomial infections. These infections encompassed postoperative pneumonia (POP), surgical site infections (SSI), and sepsis, with other infections at various body sites also taken into account.
According to the Centers for Disease Control and Prevention (CDC), SSI is defined as superficial or deep infections occurring within 30 days post-surgery22. For this study, we focus on nosocomial infections within the period of patients’ hospitalization.
The diagnosis of POP is based on the CDC’s diagnostic criteria, which include evident pneumonia or pulmonary infiltration on chest X-ray, accompanied by fever (> 38 °C), abnormal white blood cell count (< 4000/mL or > 12,000/mL), or positive blood culture results23.
Sepsis is defined by the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) as a life-threatening organ dysfunction caused by a dysregulated host response to infection, characterized by a confirmed or suspected infection and an increase in the Sequential Organ Failure Assessment (SOFA) score of 2 or more points from baseline24.
Data preprocessing
Before the analysis, the dataset was subjected to essential cleaning and preprocessing steps.Within the dataset, hgb feature exhibits a missing value rate of 0.92%, while the PT_INR, LDL_C, and Ca2 + features each have a missing value rate of 3.67%. The lac feature presents the highest rate of missing value at 4.59%. All these missing value rates are below the threshold of 5%. For addressing missing values, multiple imputation was conducted utilizing the “mice” package25, with a random forest algorithm employed to estimate the potential missing data entries. The details of missing data and the outcomes after imputation are shown in Supplementary Figure S1.
Feature selection and model development
The dataset was randomly partitioned into a training set (70%) and a validation set (30%), there are no statistically significant differences between the training set and the validation set for any variables (Supplementary Table S1). In the feature selection phase, the Boruta algorithm and LASSO algorithm were integrated to identify key variables. Initially, these algorithms were independently applied to the training set to pinpoint significant features. The intersection of features selected by both methods was then determined to ensure the importance and stability of the selected features within the model framework. Six machine learning algorithms, namely KNN, Decision Tree, Random Forest, XGBoost, LightGBM, and SVM, were engaged to assess the predictive capacity of patient data concerning postoperative infection rates. Implementation of each model was facilitated by the R language’s mlr3 package. For each machine learning algorithm, a tenfold cross-validation approach was applied on the training set to refine the algorithms and subsequently appraise their predictive efficacy. A suite of metrics were employed to evaluate the performance of each model, including classification accuracy (classif.acc), area under the receiver operating characteristic curve (classif.auc), Brier score (classif.brier), and classification error (classif.ce). The model that exhibited the most promising results on the training set was advanced for validation on the validation set.
Model interpretation
The SHAP (SHapley Additive exPlanations) framework was applied to explore the explainability of the optimal model, quantifying the influence of each feature on the model’s output and offering insights into variable significance. A variety of visualizations, such as variable importance plots, beehive plots, and variable dependence plots generated through SHAP values, were instrumental in providing an intuitive understanding of the contribution and functional mechanisms of each feature relative to the model’s output. Additionally, Local Interpretable Model-agnostic Explanations (LIME) were implemented to deliver explanations for individual predictions made by the model, particularly for continuous variables. This approach translates continuous features into binary variables based on their optimal thresholds, thereby facilitating a more granular comprehension of the decision-making process at the level of individual patients.
Statistical analysis
Demographic and initial trait comparisons among study participants were conducted utilizing the Pearson chi-square test for discrete variables and the Student’s t-test for those with a continuous nature. The Shapiro-Wilk test assessed data normality. Presented data include means with standard deviations for normally distributed continuous variables, medians with interquartile ranges for non-normally distributed ones, and frequencies or proportions for categorical data. Differences were detected through independent samples t-tests, Mann-Whitney U tests for non-parametric data, and chi-square tests as appropriate. Statistical processing was executed with SPSS software (version 26.0), with a significance level set at 0.05. Tools for data analysis and web application development comprised RStudio (version 2023.12.0), the R programming language (version 4.3.2), and SPSS (version 25).
Results
Patient characteristics
From July 1, 2011, to April 1, 2024, a total of 218 eligible patients were included in the retrospective study. Demographic and clinical characteristics of the patients at admission are detailed in Table 1. 84 cases developed postoperative nosocomial infections, with 60 diagnosed with pneumonia, 8 with surgical site infections, 10 with sepsis, and 6 with infections at other body sites, including 4 cases of abdominal infections and 2 cases of urinary system infections. There were no statistically significant differences in age, gender, smoking, drinking history and fracture site between patients with postoperative infections and those without. However, there were statistically significant differences in admission body temperature, heart rate, shock index, and GCS score between the two groups (p < 0.001, p = 0.004, p = 0.023, p < 0.001, respectively). Patients were randomly assigned to the training set (n=153) or the validation set (n=65).
Feature selection
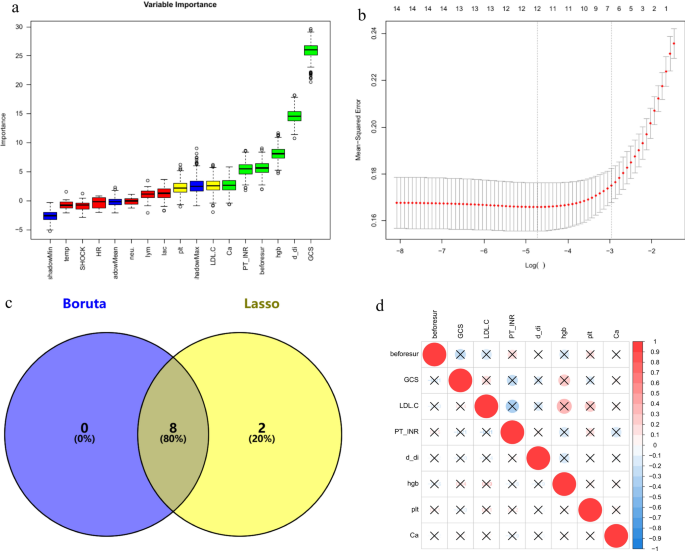
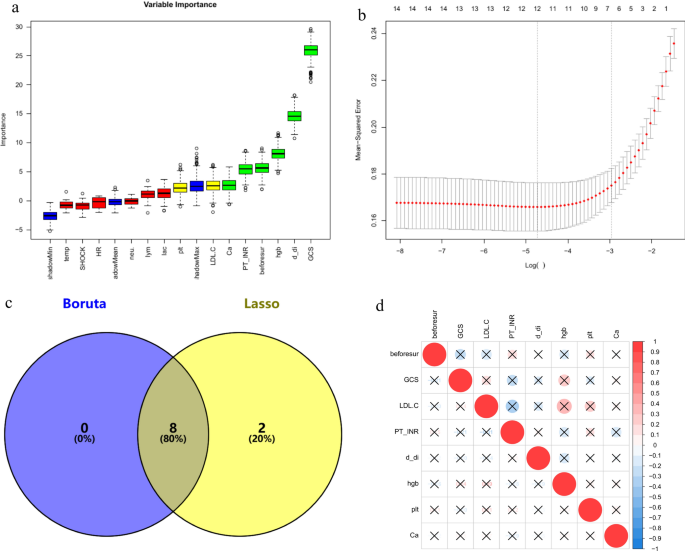
In the training set, by comparing the infected and non-infected groups, variables showing significant differences in demographic characteristics, physiological status at admission (including body temperature, heart rate, shock index, and GCS score), and non-invasive test indicators on admission and the day before surgery were subjected to feature selection using the Boruta and Lasso algorithms. The intersection of the results from both algorithms was taken, yielding a total of eight variables. These included the Glasgow Coma Scale (GCS) score, calcium (Ca), D-dimer, hemoglobin, platelet count, low-density lipoprotein cholesterol (LDL-C), prothrombin time-international normalized ratio (PT-INR), and the number of surgeries prior to the primary fracture site reconstruction. The results are illustrated using a Venn diagram (Fig. 1). Additionally, no significant collinearity was observed among these eight variables.
The process of variable elimination and assessment of multicollinearity. GCS galsgow coma scale, Hgb hemoglobin, PLT platelet, PT-INR prothrombin time-international normalized ratio, beforsur the number of surgical interventions before the repair of“major fractures”. (a) Variables are selected via the Boruta algorithm. Green and yellow represent the selected variables, red represents the variables that have been removed, and blue denotes the“shadow variables”provided by the Boruta algorithm. (b) The Lasso method is engaged in the selection process. (c) Eight variables were determined by the Boruta and Lasso algorithms. (d) A correlation matrix using either Spearman or Pearson coefficients is constructed for continuous clinical variables, with red representing positive correlations and blue representing negative correlations, and the deeper the color, the stronger the correlation. The bottom left cell of each corresponding upper right circle provides the correlation coefficient. An ‘ × ’ denotes p > 0.05, representing no statistical significance. Although some variables appear to have large correlation coefficients, they do not have statistical significance.
A multivariable logistic regression analysis was conducted on the selected eight variables. The results indicated that the GCS score (Odds Ratio, OR=0.85, p=0.011), D-dimer (OR=1.12, p=0.003), hemoglobin (OR=0.97, p=0.014), and PT-INR (OR=33.12, p=0.036) are independent risk factors for the occurrence of postoperative infection events, as detailed in Table 2.
Model performance
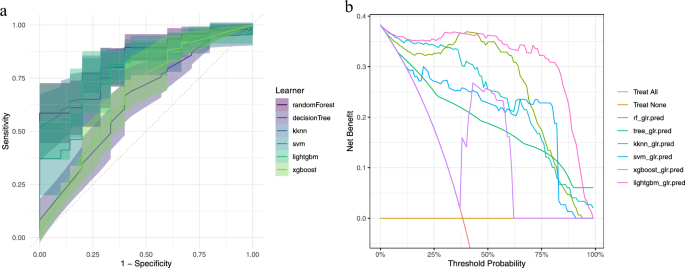
This study employed a variety of machine learning algorithms, including Random Forest, Decision Tree, K-Nearest Neighbors, Support Vector Machine, LightGBM, and XGBoost. After performing 10-fold cross-validation on the training set, we evaluated the performance metrics of each model. The results indicated that the Random Forest model exhibited the best performance on the training set with an AUC-ROC of 0.828, accuracy of 0.745, Brier score of 0.177, and classification error of 0.255. In contrast, the Decision Tree performed the worst with an AUC-ROC of 0.646. The Random Forest model demonstrated superior predictive accuracy. Table 3 presents a comparative result of the six models on the training dataset. Figure 2 illustrates the area under the receiver operating characteristic curve (AUC) and decision curve analysis (DCA) for each model.
The results of area under the receiver operating characteristic curve (AUC) and decision curve analysis (DCA) in each model. (a) ROC Curves: This graph presents the Receiver Operating Characteristic (ROC) curves for various machine learning models, assessing their ability to discriminate between patients with and without postoperative infections. The x-axis represents the false positive rate (1—Specificity), while the y-axis indicates the true positive rate (Sensitivity). The curves illustrate the trade-off between sensitivity and specificity at various threshold settings. (b) Decision Curve Analysis (DCA): The adjacent graph displays the DCA curves for the same set of models, which is a measure of the net benefit of a diagnostic test at different threshold probabilities. The x-axis shows the threshold probability, and the y-axis represents the net benefit, calculated as the difference in the proportion of patients correctly identified as having the condition versus the proportion incorrectly identified.
Validation set performance
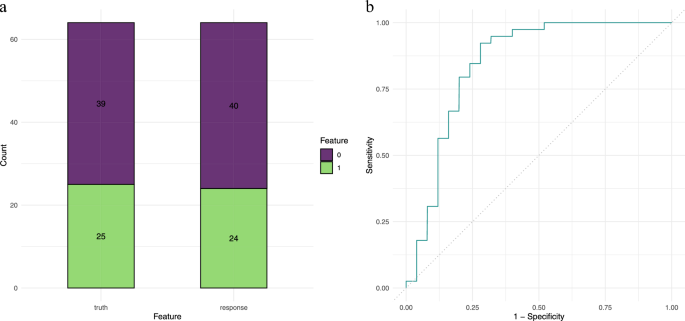
Based on the performance on the training set, the Random Forest model was selected as the optimal model. Table 4 displays the model’s performance on the validation set, which also showed good performance with a classif.auc of 0.840, classif.acc of 0.813, and classif.bbrier of 0.157, demonstrating similar excellent performance and generalization capability as seen in the training set. The confusion matrix and ROC curve (Fig. 3) present the performance of the Random Forest model on the validation set.
Model visualization and interpretation
To enhance the transparency and interpretability of the model, we utilized the SHAP algorithm for visual interpretation of the Random Forest model. For physicians and patients, understanding the importance and correlation of risk factors is a key issue that requires explanation.
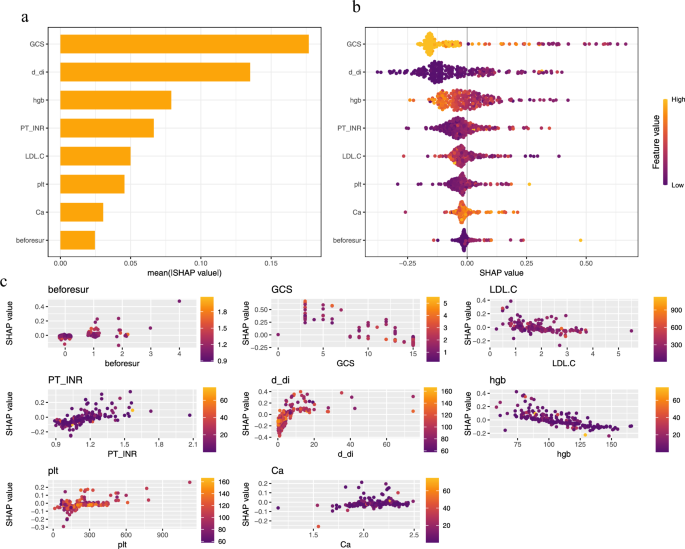
The variable importance plot (Fig. 4-a) shows that GCS, D-dimer, and hemoglobin (hgb) are the factors that most significantly affect the incidence of nosocomial infections. The contribution of the GCS score is significantly higher than that of other variables, with the widest range of SHAP values, indicating its decisive role in distinguishing between high-risk and low-risk patients. D-dimer can reflect the body’s inflammatory and coagulation status, with high levels typically associated with increased infection risk. The role of hemoglobin in oxygen transport and its association with tissue oxygenation and healing processes make it an important variable in the model.
The beeswarm plot (Fig. 4-b) further elucidates the role of each variable. A SHAP value above zero indicates an increased risk of nosocomial infection, while a value below zero suggests a reduced risk. The color of the dots represents the value of the variable. A higher GCS score (yellow) typically results in a negative SHAP value, indicating that a higher GCS score is associated with a lower risk of postoperative nosocomial infection. Higher levels of D-dimer correspond to higher SHAP values, indicating that elevated D-dimer levels are associated with increased infection risk, which is consistent with clinical experience. The mixed yellow and purple colors of hemoglobin suggest a more complex relationship between hemoglobin levels and infection risk.
The variable dependence plot (Fig. 4-c) shows the relationship between predicted risk and feature values. The plot clearly demonstrates that higher GCS scores are associated with lower infection probabilities, and that the infection risk associated with D-dimer increases nearly exponentially with concentration. Multiple surgeries may increase infection risk through cumulative physiological stress. However, the relationships between infection risk and other variables such as hemoglobin (Hgb), PT-INR, PLT, blood calcium, and LDL-C are not as intuitive in the variable dependence plot, prompting us to conduct further investigations.
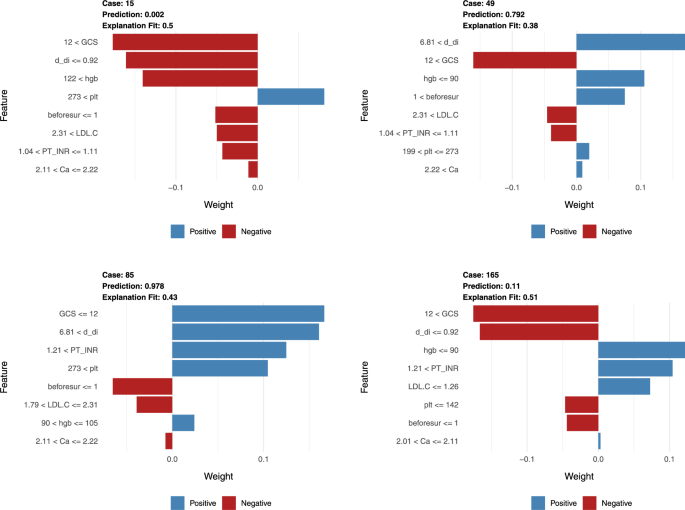
To further explore the relationship between predicted risk and feature values, we used the LIME algorithm for interpretation of continuous variables, providing unique predictive probabilities for individual samples and assessing variable importance, while also determining the predictive thresholds for key variables. Each feature’s weight is represented in blue or red, depending on whether it is beneficial to the outcome—red indicates a reduction in the risk of postoperative infection, while blue indicates an increase. Figure 5 provides four examples, showing that when the following conditions are met, patients are less likely to experience postoperative infections:
Local interpretable model-agnostic explanations (LIME) of the random forest model. This figure provides an interpretation of the random forest model’s predictions through LIME, which offers insights into how individual features influence the likelihood of postoperative infection outcomes for patients. The horizontal axis represents the impact of each feature on the probability of the outcome, with blue bars signifying features that are associated with an increased risk of postoperative infection. Conversely, red bars denote features that are linked to a decreased risk of infection. The length of each bar corresponds to the magnitude of influence on the outcome probability, quantifying the feature’s contribution as a proportion of the overall change in predicted risk.
GCS score is higher than 12 upon admission.
LDL-C levels are between 1.79 and 2.31 mmol/L upon admission.
Blood calcium levels are between 2.11 and 2.22 mmol/L upon admission.
Preoperative hemoglobin (Hgb) levels are between 105 and 122 g/L.
Preoperative prothrombin time-international normalized ratio (PT-INR) is between 1.04 and 1.11.
Preoperative D-dimer levels are less than or equal to 0.92 ug/ml.
Preoperative platelet (PLT) levels are between 142 and 273 × 10^9/L.
The number of surgical interventions prior to the repair of“major fractures”does not exceed one.
By identifying the optimal conditions under which the risk of postoperative infections is minimized, these thresholds enable a more personalized and data-driven approach to surgical planning. This method allows for the prediction of whether today is the best day for surgery based on the patient’s non-invasive test results, which is particularly valuable in the context of fluctuating timing that are often present in polytrauma patients. Through multiple measurements and predictions, combined with the experience of surgeons, the surgery date can be continuously optimized to approach the optimal surgery date, thereby reducing the occurrence of postoperative nosocomial infection events. The LIME algorithm can calculate the probability of postoperative infection from the patient’s examination results, providing a tool for surgeons to assess the surgical urgency and risk on an individual basis. This capability is crucial for making informed decisions that can improve patient outcomes and safety.
Discussion
The primary objective of this study was to employ machine learning models, in conjunction with key clinical indicators, to provide precise predictions for the optimal timing of surgery in patients with polytrauma involving traumatic brain injury and fractures. The study particularly focused on factors that may influence the risk of postoperative nosocomial infections and their role in surgical decision-making.
The Random Forest model demonstrated exceptional performance on both the training and validation datasets, underscoring the potential of machine learning methods in supporting clinical decision-making. The model’s high accuracy and AUC-ROC values indicate that the selected features are effective in distinguishing patient groups at varying risk levels. Furthermore, analysis of SHAP values allowed for the identification of key features with the most significant impact on predictive outcomes, offering valuable insights for clinicians. Visualization through SHAP and LIME revealed the relationship between the selected features and the risk of postoperative infection. Notably, certain physiological and biochemical indicators played a decisive role in the predictions. These findings highlight the importance of considering these indicators in the surgical decision-making process. The results of this study provide clinicians with a robust tool to assess the timing of surgery and potential risks, enabling the formulation of more precise individualized treatment plans through the identification of critical features and their optimal thresholds.
This study delves into the role of non-invasive indicators in the context of surgical decision-making. The Glasgow Coma Scale (GCS) score is a gold standard for assessing the severity of traumatic brain injury. A lower GCS score indicates more severe neurological damage, often associated with longer hospital stays and a more complex pathophysiological process. Current research has illuminated the crosstalk between the brain and bone, where traumatic brain injury have a positive impact on bone regeneration and fracture healing26,27. However, the co-occurrence of traumatic brain injury and fractures can exacerbate traumatic brain injury (TBI) and neuroinflammation, further worsening cerebral edema and motor disorders, thereby hindering neurological recovery28,29. Like the GCS score, the head AIS (Abbreviated Injury Scale) is also a commonly used indicator for evaluating the severity of head injuries in polytrauma patients. However, due to the absence of head AIS data in the medical records of our hospital, we were unable to include it into our study.
In the critical care of trauma patients, a low GCS score is an independent risk factor for pneumonia30. Previous studies utilizing machine learning to predict postoperative pneumonia have confirmed the significant role of GCS in such predictions31,32. Early fracture fixation in patients with head injuries does not increase the risk of neurological complications. In fact, among patients who receive early fracture stabilization, there is a quicker return to activity and a reduced risk of pulmonary complications33. An analysis of 734 TBI cases indicated that extracranial complications, such as pneumonia, sepsis, and coagulopathy, are crucial in determining the overall outcome. Early fracture stabilization and patient mobility may help reduce these extracranial complications34.
Coagulopathy at the time of admission is associated with an increased risk of complications, including pneumonia, acute renal failure, multiorgan failure, infection, sepsis, and mortality. Compared to acidosis, coagulopathy at admission is a stronger predictor of infection, sepsis, and death35. The incidence and mortality of pneumonia in patients with coagulopathy on admission are significantly higher than in those without, making the inclusion of D-dimer and PT-INR in our study meaningful. Yuan et al.36 found that approximately 18.6% of patients with traumatic brain injury exhibit coagulopathy, with D-dimer and PT-INR being closely related to the severity of brain injury. Admission PT-INR can serve as a predictive indicator of prognosis in patients with severe traumatic brain injury37. PT/INR is also a commonly used indicator to distinguish sepsis38. D-dimer is an indicator of excessive fibrinolysis in the early stages of trauma. Venous thromboembolism (VTE) is the most common diagnosis in patients with plasma D-dimer levels > 5000 ng/mL, followed by cancer and pneumonia. When plasma D-dimer levels are extremely high, the frequency of VTE, cancer, and pneumonia increases39. D-dimer is a significant risk factor for postoperative infection at the surgical site in elderly patients with intertrochanteric fractures40. A study on the impact of the trauma triad of complementopathy, endotheliopathy, and coagulopathy on clinical outcomes in severe polytrauma patients showed that elevated D-dimer levels are associated with a higher incidence of infectious complications and MOF41. Furthermore, high D-dimer levels also predict a poorer prognosis in traumatic brain injury42,43,44.
In existing research, a lower preoperative hemoglobin (Hgb) level before orthopedic surgery increases the risk of postoperative local infection45. This study incorporates preoperative Hgb levels, revealing that anemia has certain predictive value for postoperative local and systemic infections and emphasizes the significance of resuscitation in patients with multiple injuries. Additionally, Gonzalez et al.46 confirmed that anemia is a risk factor for sepsis and septic shock after hip fracture surgery. Anemia in severe traumatic brain injury increases the likelihood of pneumonia and acute lung injury ALI/ARDS47. Anemia is one of the modifiable risk factors for SSI; correcting hemoglobin may reduce the possibility of postoperative SSI48. Moreover, it has been reported that higher perioperative hemoglobin concentrations in patients with orthopedic diseases are associated with shorter hospital stays and lower mortality rates. Therefore, it is necessary to maintain patients’ hemoglobin levels at a normal or slightly higher level to reduce the incidence of SSI. The role of platelets in thrombosis is well known; excessively high platelet levels may lead to serious consequences of postoperative DVT. Recent research shows that platelets contain a large number of soluble and cell-related immune regulatory molecules, which can enhance or suppress immune responses in various environments. Platelets store pre-formed and synthesized transforming growth factor-β, interleukin-1, platelet-derived growth factor, and CC chemokine ligand 5, which can significantly affect immune responses. Platelets express Toll-like receptors and actively bind to microorganisms, sometimes directly causing cytopathic effects on bacteria49. Salehpour et al.’s research also shows that platelet count is positively correlated with the GCS and GOS scores of patients with severe traumatic brain injury, suggesting that platelets may have a protective effect on the neurological function of patients with severe traumatic brain injury.
Calcium (Ca) plays a pivotal role in coagulation, cardiac output, and peripheral vascular resistance. It is a key component of the trauma resuscitation and coagulation cascade, reflecting, to some extent, the effectiveness of resuscitation in patients with multiple injuries50. The addition of hypocalcemia to the“lethal triad”of hypothermia, acidosis, and coagulopathy forms the"diamond of death"in trauma51. A significant proportion (85% to 94%) of trauma patients undergoing massive transfusion exhibit hypocalcemia52, which is intrinsically linked to the negative outcomes associated with the components of the lethal triad. Hypocalcemia has both direct and indirect effects on each aspect of the lethal triad, supporting the potential role of calcium as the fourth component in the proposed diamond of death. Hypocalcemia is a common occurrence in trauma patients following severe bleeding associated with injury, and necessary transfusions and resuscitation efforts can exacerbate hypocalcemia. The serious consequences of hypocalcemia in trauma patients have been repeatedly demonstrated, with associated morbidity and mortality51. An analysis of the TraumaRegister DGU database from a multicenter cohort revealed a parabolic relationship between non-dependent ionized calcium (iCa2 +) levels and coagulation dysfunction, transfusion requirements, and mortality in major trauma patients. Both hypocalcemia and hypercalcemia are independently associated with coagulation disorders and transfusion53.
Low-density lipoprotein cholesterol (LDL-C) affects hematopoiesis, promoting the quantity of circulating monocytes and pro-inflammatory activation54. A study focusing on patients with low-energy femoral neck fractures indicated that elevated LDL-C may lead to avascular necrosis of the femoral head55. In elderly patients with hip fractures, both high and low levels of LDL-C are associated with increased mortality rates and reduced ability to walk independently56. An epidemiological observation and Mendelian randomization study confirmed a negative causal relationship between LDL-C levels and bone mineral density (BMD)57. Another Mendelian randomization study examining the causal relationship between plasma lipids and sepsis identified elevated LDL-C as a risk factor for sepsis58. Incorporating LDL-C into predictive models for postoperative infection holds potential value.
The number of surgical interventions prior to the repair of“major fractures”reflects, to some extent, the extent of damage to other secondary areas of the patient’s body, including the brain, bones, and soft tissues. Our study supports the notion of minimizing the number of surgeries for major trauma. According to the"two-hit"hypothesis, multiple surgeries may increase physiological stress and immune suppression in patients, thereby increasing the risk of infection. In addition to the fracture site, associated diseases and injuries can also affect the decision-making for the definitive fixation of"major fractures."Each surgery may introduce new sources of infection. A lower number of surgeries is associated with a reduced risk of surgery-related complications and improved overall patient prognosis59,60.
This study is a single-center retrospective study that relies on existing medical records and data. Although we have carefully selected key variables through a rigorous screening process, the limitations in sample size may have affected the model’s generalizability and may not have covered all clinically relevant factors affecting the timing of surgery and the risk of postoperative infection. The lack of detailed descriptions of patient in the medical records caused us to give up further analysis of TBI (Traumatic Brain Injury) types, which diminished the accuracy of our model’s analysis for specific TBI injury cases. However, this was beneficial in terms of the model’s general applicability. Future studies should consider prospectively collecting more comprehensive data, including head AIS and detailed classifications of TBI, to enhance the model’s ability to interpret individual characteristics of TBI patients. The absence of descriptions of ISS and AIS scores in the medical records precluded us from incorporating these two indicators into the study. We acknowledge that injury characteristics and other time features may represent potential limitations to the analysis, but we believe that the selected variables effectively reflect the physiological status of patients and provide valuable information for clinical decision-making. Multiple injuries are complicated. However, if too many factors are considered, the model will have the defects of overfitting and bloating, which will weaken the value of this study. Predictive models can bring convenience and assistance to surgeons, but the professional judgment of surgeons should not be ignored. Therefore, we tried our best to incorporate the fewest and most effective predictor variables in this study. The lack of external validation further limits the universality of our results, necessitating further testing of the model’s predictive performance on an independent dataset. The insufficiency of long-term follow-up data also limits the assessment of patients’ long-term prognosis and recovery. Therefore, future research should adopt a prospective design, expand the sample size, conduct multicenter validation, and consider more comprehensive clinical variables and long-term follow-up to enhance the accuracy and practicality of the model.
Guided by the"Safe Definitive Orthopaedic Surgery (SDS)"principle61, we emphasize the importance of performing fracture repair surgery as soon as possible after adequate resuscitation has stabilized the patient’s vital signs to reduce pulmonary, local, and systemic infectious complications. Utilizing machine learning models, we can promptly assess the physiological status of patients and proceed with fracture repair surgery in a timely manner. Moreover, the non-invasive indicators included in our predictive model are easily obtained clinically, providing tangible assistance for clinical decision-making for physicians.
Data availability
The datasets generated during and/or analysed during the current study are not publicly available due to legal and privacy issues but are available from the corresponding author on reasonable request.
References
Morshed, S. et al. Delayed internal fixation of femoral shaft fracture reduces mortality among patients with multisystem trauma. J. Bone Joint Surg.-Am. 91, 3–13 (2009).
Zheng, J. et al. Early vs Late Fixation of Extremity Fractures Among Adults With Traumatic Brain Injury. JAMA Netw. Open 7, e241556 (2024).
Nahm, N. J. & Vallier, H. A. Timing of definitive treatment of femoral shaft fractures in patients with multiple injuries: A systematic review of randomized and nonrandomized trials. J. Trauma Acute Care Surg. 73, 1046–1063 (2012).
Giannoudis, P. V., Veysi, V. T., Pape, H.-C., Krettek, C. & Smith, M. R. When should we operate on major fractures in patients with severe head injuries?. Am. J. Surg. 183, 261–267 (2002).
Grotz, M. R. W. et al. Traumatic brain injury and stabilisation of long bone fractures: An update. Injury 35, 1077–1086 (2004).
Dvorak, J. E., Lasinski, A. M., Romeo, N. M., Hirschfeld, A. & Claridge, J. A. Fracture related infection and sepsis in orthopedic trauma: A review. Surgery S0039–6060(24), 00283–00286. https://doi.org/10.1016/j.surg.2024.04.031 (2024).
Li, J. et al. Incidence and risk factors for surgical site infection following open reduction and internal fixation of adult tibial plateau fractures. Int. Orthop. 42, 1397–1403 (2018).
Lowe, J., Mitchell, S. M., Agarwal, S. & Jones, C. B. Traumatic Hip Fracture and Primary Elective Total Hip Patients are Not the Same: A Comparison of Comorbidity Burden, Hospital Course, Postoperative Complications, and Cost of Care Analysis. J .Orthop. Trauma 34, 583–588 (2020).
Brundage, S. I., McGhan, R., Jurkovich, G. J., Mack, C. D. & Maier, R. V. Timing of femur fracture fixation: Effect on outcome in patients with thoracic and head injuries. J. Trauma 52, 299–307 (2002).
Onizuka, N. et al. Timing of Complications Following Surgery for Distal Femur Fractures in Older Adults. Geriatr. Orthop. Surg. Rehabil. 14, 21514593231195540 (2023).
Sun, L., Wang, C., Zhang, M., Li, X. & Zhao, B. The Surgical Timing and Prognoses of Elderly Patients with Hip Fractures: A Retrospective Analysis. Clin. Interv. Aging 18, 891–899 (2023).
Schreiber, V. M. et al. The timing of definitive fixation for major fractures in polytrauma–a matched-pair comparison between a US and European level I centres: Analysis of current fracture management practice in polytrauma. Injury 42, 650–654 (2011).
Pape, H. C. & Pfeifer, R. Safe definitive orthopaedic surgery (SDS): repeated assessment for tapered application of Early Definitive Care and Damage Control?: An inclusive view of recent advances in polytrauma management. Injury 46, 1–3 (2015).
Kalbas, Y. et al. Developments in the understanding of staging a ‘major fracture’ in polytrauma: Results from an initiative by the polytrauma section of ESTES. Eur. J. Trauma Emerg. Surg. 50, 657–669 (2024).
Giannoudis, V. P., Rodham, P., Giannoudis, P. V. & Kanakaris, N. K. Severely injured patients: Modern management strategies. EFORT Open Rev. 8, 382–396 (2023).
Kalbas, Y. et al. Fracture fixation in polytraumatized patients-From an interdisciplinary early total/appropriate care to the safe definitive surgery concept. Front .Med. (Lausanne) 11, 1362986 (2024).
Arnold, S. C. et al. Two big bones, one big decision: When to fix bilateral femur fractures. Injury 55, 111610 (2024).
Papakostidis, C., Panagiotopoulos, A., Piccioli, A. & Giannoudis, P. V. Timing of internal fixation of femoral neck fractures. A systematic review and meta-analysis of the final outcome. Injury 46, 459–466 (2015).
Padash, S. et al. An Overview of Machine Learning in Orthopedic Surgery: An Educational Paper. J. Arthroplasty 38, 1938–1942 (2023).
Elfanagely, O. et al. Machine Learning and Surgical Outcomes Prediction: A Systematic Review. J. Surg. Res. 264, 346–361 (2021).
Gutierrez-Naranjo, J. M. et al. A machine learning model to predict surgical site infection after surgery of lower extremity fractures. Int. Orthop. 48, 1887–1896 (2024).
Surgical Site Infections (SSI) | OPC | NHSN | CDC. https://www.cdc.gov/nhsn/opc/ssi/index.html (2024).
Abbott, T. E. F. et al. A systematic review and consensus definitions for standardised end-points in perioperative medicine: Pulmonary complications. Br. J. Anaesth. 120, 1066–1079 (2018).
Singer, M. et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315, 801–810 (2016).
van Buuren, S. & Groothuis-Oudshoorn, K. Mice: Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 45, 1–67 (2011).
Otto, E. et al. Crosstalk of Brain and Bone-Clinical Observations and Their Molecular Bases. Int. J. Mol. Sci. 21, 4946 (2020).
Liu, W. et al. Traumatic brain injury stimulates sympathetic tone-mediated bone marrow myelopoiesis to favor fracture healing. Signal Transduct. Target Ther. 8, 260 (2023).
Paul, M. M. et al. Impact of a Femoral Fracture on Outcome after Traumatic Brain Injury-A Matched-Pair Analysis of the TraumaRegister DGU®. J. Clin. Med. 12, 3802 (2023).
Shultz, S. R. et al. Tibial fracture exacerbates traumatic brain injury outcomes and neuroinflammation in a novel mouse model of multitrauma. J. Cereb. Blood Flow Metab. 35, 1339–1347 (2015).
Hyllienmark, P. et al. High incidence of post-injury pneumonia in intensive care-treated trauma patients. Acta Anaesthesiol. Scand. 57, 848–854 (2013).
Jin, X. et al. Development and external validation of a nomogram for predicting postoperative pneumonia in aneurysmal subarachnoid hemorrhage. Front. Neurol. 14, 1251570 (2023).
Hsu, S.-D. et al. Machine Learning Algorithms to Predict In-Hospital Mortality in Patients with Traumatic Brain Injury. J. Pers. Med. 11, 1144 (2021).
Kushwaha, V. P. & Garland, D. G. Extremity fractures in the patient with a traumatic brain injury. J. Am. Acad. Orthop. Surg. 6, 298–307 (1998).
Lu, S. et al. Timing of Extremity Fracture Fixation in Patients with Traumatic Brain Injury: A Meta-Analysis of Prognosis. World Neurosurg. 133, 227–236 (2020).
Childs, B. R., Verhotz, D. R., Moore, T. A. & Vallier, H. A. Presentation Coagulopathy and Persistent Acidosis Predict Complications in Orthopaedic Trauma Patients. J. Orthop. Trauma 31, 617–623 (2017).
Yuan, Q. et al. Prognostic value of coagulation tests for in-hospital mortality in patients with traumatic brain injury. Scand. J. Trauma Resusc. Emerg. Med. 26, 3 (2018).
Salehpour, F., Bazzazi, A. M., Porhomayon, J. & Nader, N. D. Correlation between coagulopathy and outcome in severe head trauma in neurointensive care and trauma units. J. Crit. Care 26, 352–356 (2011).
Schupp, T. et al. Diagnostic and Prognostic Significance of the Prothrombin Time/International Normalized Ratio in Sepsis and Septic Shock. Clin. Appl. Thromb. Hemost. 28, 10760296221137892 (2022).
Schafer, K. et al. The clinical significance of ultra-high D-dimer levels. J. Vasc. Surg. Venous. Lymphat. Disord. 10, 8–13 (2022).
Hazucha, M. J. Relationship between ozone exposure and pulmonary function changes. J. Appl. Physiol. 1985(62), 1671–1680 (1987).
Yang, Z. et al. Traumatized triad of complementopathy, endotheliopathy, and coagulopathy - Impact on clinical outcomes in severe polytrauma patients. Front. Immunol. 13, 991048 (2022).
Hayakawa, M. et al. High D-Dimer levels predict a poor outcome in patients with severe trauma, even with high fibrinogen levels on arrival: A multicenter retrospective study. Shock 45, 308–314 (2016).
Kuo, J.-R. et al. Correlation of a high D-dimer level with poor outcome in traumatic intracranial hemorrhage. Eur. J. Neurol. 14, 1073–1078 (2007).
Nakae, R. et al. Neurointensive Care of Traumatic Brain Injury Patients Based on Coagulation and Fibrinolytic Parameter Monitoring. Neurol. Med. Chir. (Tokyo) 62, 535–541 (2022).
McLaren, A. C. & Lundy, D. W. AAOS Systematic Literature Review: Summary on the Management of Surgical Site Infections. J. Am. Acad. Orthop. Surg. 27, e717–e720 (2019).
Gonzalez, C. A. et al. Postoperative sepsis and septic shock after hip fracture surgery. Injury 54, 110833 (2023).
Schirmer-Mikalsen, K. et al. Severe head injury: Control of physiological variables, organ failure and complications in the intensive care unit. Acta Anaesthesiol. Scand. 51, 1194–1201 (2007).
Huang, X., Guo, Y., Fu, R. & Li, H. A nomogram to predict postoperative surgical site infection of adult patients who received orthopaedic surgery: A retrospective study. Sci. Rep. 13, 8129 (2023).
Semple, J. W., Italiano, J. E. & Freedman, J. Platelets and the immune continuum. Nat. Rev. Immunol. 11, 264–274 (2011).
Vasudeva, M. et al. Hypocalcemia in trauma patients: A systematic review. J. Trauma Acute Care Surg. 90, 396–402 (2021).
Wray, J. P. et al. The diamond of death: Hypocalcemia in trauma and resuscitation. Am. J. Emerg. Med. 41, 104–109 (2021).
Wade, D. J. et al. Higher Doses of Calcium Associated With Survival in Trauma Patients. J. Surg. Res. S0022–4804(24), 00094–00095. https://doi.org/10.1016/j.jss.2024.02.014 (2024).
Chai, S. et al. Internal validation of an improved system for forensic application: A 41-plex Y-STR panel. Forensic. Sci. Res. 8, 70–78 (2023).
Stiekema, L. C. A. et al. Impact of cholesterol on proinflammatory monocyte production by the bone marrow. Eur. Heart J. 42, 4309–4320 (2021).
Zhang, C. et al. Dyslipidaemia for patients with low-energy femoral neck fractures after the treatment of cancellous screws: A retrospective study with a 3-year minimum follow-up. BMC Musculoskelet Disord. 18, 440 (2017).
Zhao, Z., Fan, W., Wang, L. & Chu, Q. The Paradoxical Association of Lipids with Survival and Walking Ability of Hip Fractures in Geriatric Patients After Surgery: A 1-Year Follow-Up Study. Int. J. Gen. Med. 16, 3907–3919 (2023).
Li, G.H.-Y. et al. Positive effects of low LDL-C and statins on bone mineral density: an integrated epidemiological observation analysis and Mendelian randomization study. Int. J. Epidemiol. 49, 1221–1235 (2020).
Chen, J. et al. Causal relationships between plasma lipids and sepsis: A Mendelian randomization study. Medicine (Baltimore) 102, e36288 (2023).
Klingebiel, F.K.-L. et al. Surgical load in major fractures - results of a survey on the optimal quantification and timing of surgery in polytraumatized patients. Int. Orthop. 47, 1677–1687 (2023).
Kalbas, Y. et al. Developments in the understanding of staging a ‘major fracture’ in polytrauma: Results from an initiative by the polytrauma section of ESTES. Eur. J. Trauma Emerg. Surg. https://doi.org/10.1007/s00068-023-02245-5 (2023).
Kalbas, Y. et al. Fracture fixation in polytraumatized patients-From an interdisciplinary early total/appropriate care to the safe definitive surgery concept. Front. Med. (Lausanne) 11, 1362986 (2024).
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Han, X., Zhang, JH., Zhao, X. et al. Interpretable machine learning for predicting optimal surgical timing in polytrauma patients with TBI and fractures to reduce postoperative infection risk. Sci Rep 15, 18347 (2025). https://doi.org/10.1038/s41598-025-04003-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-04003-6
关于《Interpretable machine learning for predicting optimal surgical timing in polytrauma patients with TBI and fractures to reduce postoperative infection risk》的评论
发表评论
摘要
相关新闻
相关讨论
- [上海][乱谈会]11月30日周日上海乱谈会特别活动《乱谈读书会第一期》通知
- 刷Leetcode (01) Remove Duplicates from Sorted Array
- 在新加坡做面试官的经历 (Interviewer Experience for UI/UX Designer in Singapore)
- 学习笔记TF066:TensorFlow移动端应用,iOS、Android系统实践
- Global Neck Cream and Mask Sales Market Surges with Demand for Targeted Skincare Solutions and Anti-Aging Innovations