特发性肺纤维化的生成性AI下发现的TNIK抑制剂:一项随机2A期试验
作者:Zhavoronkov, Alex
主要的
开发新型治疗剂的过程越来越缓慢且昂贵1,在10 15年的时间里,平均花费了23亿美元,将新药推向市场2。人工智能(AI)的进步已证明了概念验证的创新,并通过促进与疾病相关的目标优先级来加速和降低制药研究和开发各个阶段成本的潜力3,,,,4,,,,5,复合设计和优化6,,,,7,,,,8,,,,9,,,,10和临床试验性能11,,,,12,,,,13,,,,14,,,,15。将更多的时间,物质和劳动力的药物发现步骤转移到AI平台上,可以更快地提名治疗候选化合物,以更彻底地探索可能的化学空间16,,,,17并随之而来的动手筛选18,,,,19,从而使更多的临床前候选化合物可以进入临床测试,并最终进行临床实践。
尽管有这些进步,但很少有AI发现或AI设计的药物已经进行了临床试验。AI发现的药物经历了与未发现药物相似的2期试验失败水平20,,,,21,到目前为止,在第三阶段试验中没有任何进展21。人工智能是否可以赋予有意义的,持续破坏药物开发的问题尚未得到答复21。
作为对这个问题的答案,我们的小组使用了生成AI驱动的发现工具22,,,,23鉴定traf2-和NCK相互作用激酶(TNIK)de Novo作为特发性肺纤维化(IPF)病理学的关键调节剂,因为它策划了多种纤维化和促进性细胞程序9。我们的方法导致了第一个抗纤维化的小分子TNIK抑制剂Rentosertib(以前是ISM001-055),用于治疗由生成AI设计的IPF。我们报道了Rentosertib的开发和积极的0和1试验结果9((NCT05154240和CTR20221542(通过http://www.chinadrugtrials.org.cn/),第一份针对性的TNIK抑制剂进入临床测试的报告,发现Rentosertib在两个试验中都表现出健康个体的安全和耐受性,表现出良好的药代动力学(PK)。9。重要的是,这也是第一个报道的以AI平台为支持疾病相关的靶标和该靶标的化合物的实例。我们的生成AI驱动方法将临床前候选者提名简化为18个月,并完成0/1临床测试,从启动目标发现后的30个月以下,代表了药物发现的精简革命性转变9。
IPF是一种与年龄相关的,进行性肺部疾病,可通过排除其他潜在的间质性肺疾病(例如,环境暴露,结缔组织疾病,超敏性肺炎或药物毒性)来诊断出。24并以间质肺炎,呼吸困难和咳嗽为标志25,组织学以成纤维细胞增殖和细胞外基质重塑为特征26,,,,27,,,,28,,,,29。在美国影响每100,000个个人10至60人之间,在65岁以上的人中发生了大约十倍,IPF的发生率类似于胃,大脑和睾丸癌25。尽管有针对性的抗纤维化疗法,诊断后的中位生存时间为2年4年26,,,,30,强调对治疗IPF的新颖,有效疗法的持续需求。治疗IPF的当前护理标准(SOC)治疗方案包括nintedanib,nintedanib是受体酪氨酸激酶的广泛作用抑制剂31,或Pirfenidone,TGFî²介导的成纤维细胞到肌纤维细胞过渡的抑制剂32。研究IPF患者的这两种药物的临床试验仅显示出疾病进展的放缓33,,,,34,通过强制生命能力(FVC)和自我报告的生活质量(QOL)指标以及生存的不清楚的好处26,使可以恢复肺功能并将疾病进程的疗法发展为优先事项。
在这里,我们介绍了2A期多中心,双盲,随机,安慰剂对照试验的试验,以评估IPF患者的一系列剂量的安全性,耐受性,PK和对Rentosertib的FVC的影响。设计了其他二次和探索性终点和分析,以进一步衡量与Rentosertib治疗和反应相关的肺功能,QOL指标和细胞表型。
结果
患者
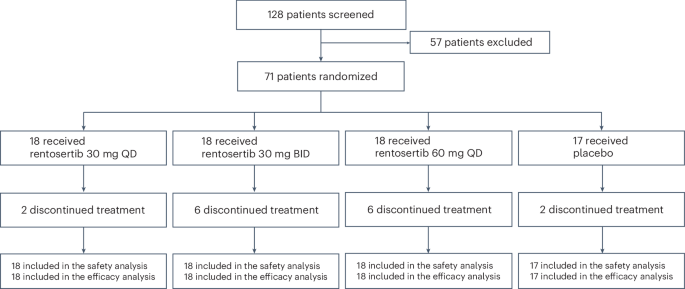
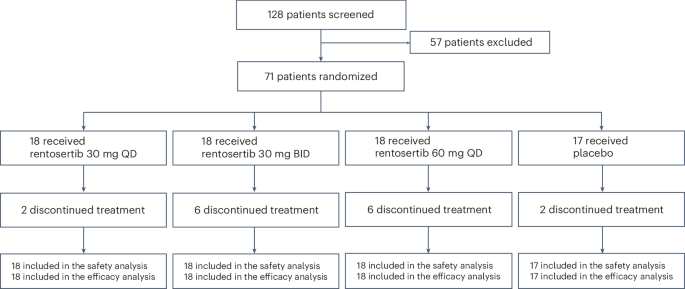
在筛查纳入的128名患者中,有71名(55.5%)患者被招收并随机分配接受安慰剂(n= 17),每天30毫克Rentosertib(QD,n= 18),每天两次30毫克Rentosertib(bid,n= 18)或60 mg rentosertib QD(n= 18)(扩展数据图。1)。基于筛查的排除的最常见原因是阻塞性肺部疾病合并症是由1 s(FEV1)/FVC强迫到期1(基线)(基线)或肺部肺部碳(DLCO)(DLCO)肺部肺部(DLCO)占基线范围内预测的正常范围的百分比小于0.7的0.7的最常见原因。在访问1(基线)或访问2(第1周)的近期上或下呼吸道感染(在4周内)的近期病史(第1周)中,患者也被排除在外。意向治疗的人群包括71名患者,55例(77%)完成了为期12周的安慰剂对照期(安慰剂组中的15(88%),在30毫克Rentosertib QD组中,有16个(89%),在30毫克Rentosertib BID组中,12(67%)在60英里(67%)中,在60毫克的租金中,并在60毫克租金组中。总体而言,由于不良事件(AES)或其他戒断,在12周研究结束之前,有16名(22.5%)的患者停止治疗;2来自安慰剂,其中2个,来自30毫克Rentosertib QD,30毫克Rentosertib竞标的6个,60毫克Rentosertib QD组的6个。所有患者都纳入了安全性和功效分析中(图。1)。图1:试验参与者随机和随访方案。在128例筛选纳入的患者中,有71例被随机分配接受30 mg rentosertib QD(
= 18),30 mg rentosertib竞标(n= 18),60 mg rentosertib QD(n= 18)或安慰剂(n= 17)在12周的过程中。16名患者在治疗结束之前停止治疗。源数据临床和人口统计学特征,包括年龄,体重指数(BMI)和基线肺功能,在治疗组之间相似(表1)。表1基线患者的特征
在所有治疗组中,治疗效果AES(TEAE)的速率相似(30毫克Rentosertib QD组中的13/18(72.2%),在30毫克Rentosertib bid组中,15/18(83.3%),15/18(83.3%)在60毫克Rentosertib QD Group和12/6%(70.6%(70.6%)中(83.3%)2),患者同时服用SOC抗纤维化疗法的发生率相似(补充表
)。
与安慰剂相比,接受Rentosertib的患者与治疗相关的AES更为普遍,安慰剂组为5/17(29.4%),而30毫克QD组中的9/18(50.0.0%)为9/18(50.0%),11/18(61.1%)在30毫克BID组中,在30毫克BID组中,14/18(77.8%)(77.8%)中的60 h 60 h. 60 ount in 60 ount。其中,很少有人是严重的AE(SAE),安慰剂组中没有与治疗相关的SAE,rentosertib 30 mg QD组的1/18(5.6%),在30毫克BID组中2/18(11.1%)和2/18(11.1%)(11.1%)(11.1%)(桌子QD组)2)。最常见的茶是低钾血症(安慰剂2(11.8%),30毫克QD中的3个(16.7%),30毫克bid中的5个(27.8%)(27.8%)和60毫克QD(20.4%)中的3分(20.4%)),肝功能异常(2 intbo(11.8%),230.8%),30.1%的Mg bg qD(11.1%),4.1%Mg qD QD,4(22.2%),腹泻(安慰剂中0,30毫克QD(11.1%),30毫克bid中的3个(16.7%),60毫克QD(27.8%))和丙氨酸氨基转移酶(Alt)(Alt)(替代)增加(1 n 30%),30%(5.6%)(5.6%)(5.6%)(5.6%),Mg bid(5.6%),Mg bid(5.6%),bid(5.6%);在60毫克QD中(33.3%)和30毫克的QD(16.7%)。2。表2 AE治疗期间治疗中断的最常见原因是AE(12/16(75%)),并且在30毫克QD组中,由于肝损伤或功能障碍而导致的12例患者中7例的治疗因肝损伤或功能障碍而导致AES中停产(0/18(0%),4/18(22.2%)在30毫克bid BID组和30%(22.2%)中(22.2%)(22.2%),并在60%(17%)中(17%)(17%)(17%)(17%)(17%)(17%)(
)。由于肝脏毒性而撤回的七个(57.1%)中有四名同时服用了Nintedanib抗纤维化疗法。剩余的四个(75%)中的三个中断是由于患者退出试验(3/71例(4.2%))。一名患者因退化性主动脉瓣疾病,主动脉瓣瓣膜功能不合,左心室功能障碍,主动脉膨胀,肺部膨胀,肺部高血压增加,血浆和超胆固醇症状以及其他缺乏,以及其他缺乏的note vrose and vrose and vrose and vrose and vrose and vose,一名患者死于第十二周治疗(EOT)就诊(EOT)。自随机分组以来,检查,12铅心电图(ECG)或实验室测试。在补充表中介绍了由于TEAE而停止治疗的患者的完整清单4。次级肺功能终点FVC是评估IPF患者肺功能和对治疗干预措施的肺功能的金标准度量
33
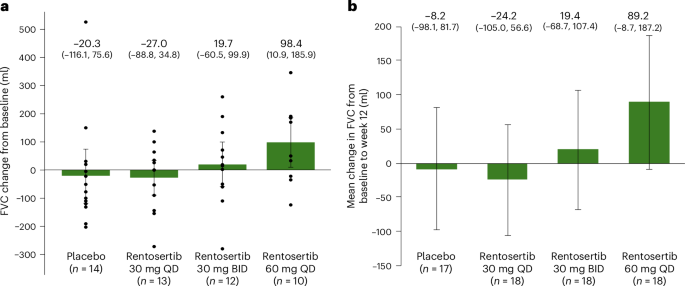
,,,,34,,,,35。经过12周的治疗后,接受安慰剂的患者的平均变化为20.3 mL(95%置信区间(CI)116.1至75.6)。接受30毫克Rentosertib QD的患者的FVC平均降低相似,为27.0毫升(95%CI -88.8至34.8),接受30毫克Rentosertib竞标的患者的平均变化经历了 +19.7毫升的平均变化 +19.7 ml(95%CI ci – 60.5至99.9),并与60.5至99.9的患者搭配60.9),并伴有60.9的患者,并伴随着60.9的患者,并有效。+98.4毫升(95%CI 10.9至185.9)(图。2并扩展数据图。2)。接受60毫克Rentosertib QD的患者不同时接受SOC抗纤维化疗法的FVC(+187.8毫升,95%CI 68.6至306.9 ml)的表现显着改善,而患者同意使用NINTEDANIB或PIRFENID ins PROTAGE SREABOR Survience Survision(fvc),患者同意接受60毫克Rentosertib qd。3)。图2:与基线相比,Rentosertib治疗12周后FVC的变化95%CI的变化。一个
自我报告的QOL和身体能力指标的变化,例如对莱斯特咳嗽问卷(LCQ)和6分钟步行距离(6MWD)的反应(6MWD),同样在治疗组之间相似(扩展数据图。4fâi),尽管我们的建模表明,与接受安慰剂相比,接收60 mg rentosertib的患者的LCQ评分的最小二乘平均变化显着增加p= 0.0495)。三名患者(16.7%)接受60毫克Rentosertib QD和一名患者(5.9%)接受安慰剂的患者经历了IPF(AE-IPF)的急性加重(补充表5)。
60毫克QD组的患者因急性加重而住院,平均持续时间为23.3天,而安慰剂组的患者则未住院。事后探索性分析药代动力学
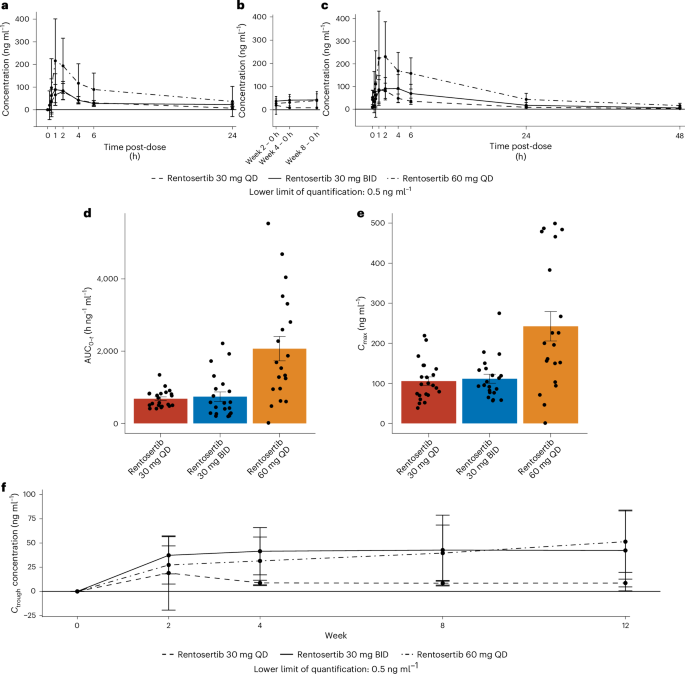
单剂量和多剂量后,IPF患者的血浆中的Rentosertib浓度的PK分析显示出良好的PK特征,与接受30毫克Rentosertib QD或30毫克Rentosertib QD或30毫克Rentosertib bid的患者相比,接受60 mg rentosertib QD的患者的暴露率更高,在给药后达到峰值1小时(图(图)。
3aâc
和补充表6)。等离子体浓度曲线下的面积(AUC0t),在第0周(首次剂量)和第12周(eot;算术平均值1,630和3,450)的60毫克Rentosertib QD中测量对药物的总暴露量更大1ml1分别比30毫克的Rentosertib竞标(315和1,3901ml1)和30毫克Rentosertib QD(553和788hâng1ml1) (如图。3D,扩展数据图。5a和补充表6)。在第2周实现了稳态,在第12周之前未观察到明显的药物积累。几何平均半衰期(t1/2)在第12周的10.9到12.0 h。图3:Rentosertib的药代动力学特性。一个
,在第0周的24 h治疗期间,predose的患者血清中的Rentosertib的药代动力学动力学(一个),在第2、4和第8周进行治疗后0 h(b)和第12周管理后的48 h(c)。d,AUC0tRentosertib的暴露;n= 30毫克QD中的22n= 30毫克竞标中的21n= 60毫克QD中的20分。e,,,,c最大限度Rentosertib的暴露;n= 30毫克QD中的22n= 30毫克竞标中的21n= 60毫克QD中的20分。f,,,,c槽在第0、2、4、8和12周收集的患者血清中的Rentosertib暴露。所有数据均代表算术平均值。更高的AUC0t和t1/2所有剂量的第12周都表明,随着治疗时间的增加,对Rentosertib的接触增加(图。3a,c并扩展数据图。5a,b)。
患者FVC从基线到第12周的变化与AUC正相关0t和谷浓度(c槽)(扩展数据图。5C,d)。毫不奇怪,c最大限度接受60 mg QD剂量的患者的暴露最大,30毫克QD和30毫克竞标表现出类似的较低c最大限度(如图。3e), 然而c槽在30 mg竞标和60 mg QD剂量之间相似(图。3f)。总之,PK动力学表明,较高的剂量会导致较高初始净吸收的暴露量增加,并且在治疗期结束时与清除较慢有关的治疗期结束。血液反应的生物标志物和蛋白质组学特征我们对患者血清样品进行了蛋白质组学分析,以了解Rentosertib的作用机理,指导反应的生物标志物的发展,并评估抑制TNIK可能针对生物衰老的假设36。我们使用Olink探索了3072个面板,跨基线,2周,4周和12周,我们使用Olink探索3072张面板确定了2,841种蛋白质。使用线性回归模型来评估Rentosertib治疗对蛋白质表达变化的时间依赖性影响,我们发现治疗持续时间基本上影响了蛋白质表达模式,分别在第2、4和12周的治疗中分别使用20、82和192蛋白质(Benjamini hochberg-hochberg-djjusted)p值<0.1)(扩展数据图。
6a
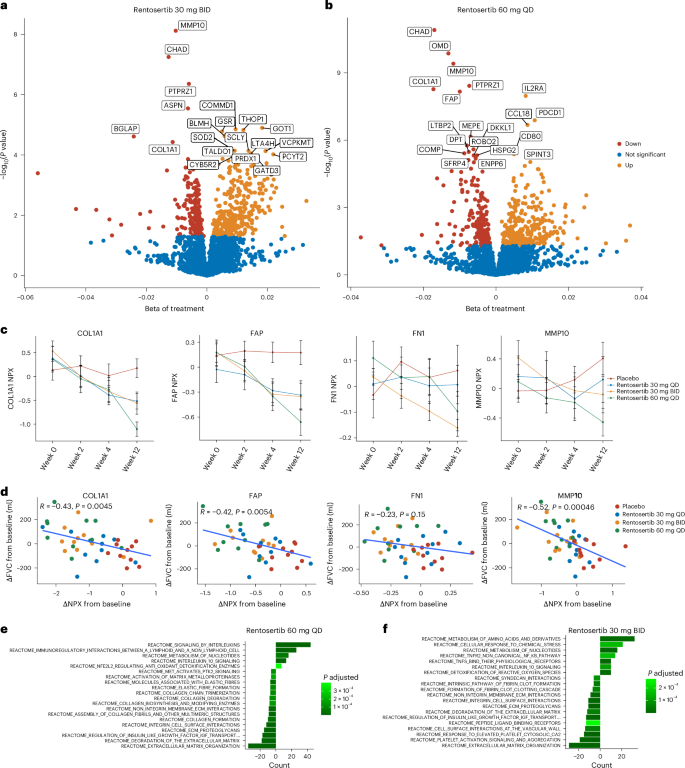
)。与确定的安慰剂相比,配对统计测试以评估蛋白质表达的剂量相关变化padj<0.05)分别使用广义线性模型分别接受30 mg QD,30毫克bid和60 mg Qd rentosertib的患者的血清中差异丰富的蛋白质(图。4a,b和补充表79)。从基线到第12周的差异蛋白分析的限制,显着改变的蛋白质的数量大大增加,较高的给药:在30 mg QD,30 mg QD,30 mg BID和60 mg QD组中,蛋白质的数量大大增加:扩展数据。6b)。总之,这表明Rentosertib治疗和剂量的持续时间与血清蛋白谱的变化有关。图4:血清蛋白分析的事后探索性分析。一个,,,,b,在30毫克bid的患者的血清中差异丰富的蛋白质(一个
b)用广义线性模型识别。c,Col1a1,FAP,FN1和MMP10的血清水平随Rentosertib剂量和治疗时间而降低。n=安慰剂中的11名患者,n= 30 QD中的11例患者,n= 30名出价的11例患者,n= 60 QD中的10例患者。d,从基线到第12周的COL1A1,FAP,FN1和MMP10的血清水平的变化与FVC从基线到第12周的变化成反比。n每次访问时有43名患者;p通过Pearson相关分析计算的价值。e,,,,f,反应组的途径富集在30 mg bid的患者的血清中差异丰富的蛋白质(e)和60 mg QD(f)。与30毫克BID和60毫克QD处理相关的下调蛋白质包括已知的纤维化相关蛋白,例如MMP10,PTPRZ1,COL1A1,FAP,FN1,ROBO2,ASPN和LTBP2(图。4aâc和补充表710)。分析差异丰富的蛋白质发现与FVC变化的关联发现,ASPN,PTPRZ1,MMP10和CHAD都与FVC的变化显着相关(p<0.05),在第12周的时间点给予伦瑟替伯后显着下调,这表明有一系列潜在的生物标志物可以响应治疗(图。4aâd
和补充表79)。途径富集分析确定细胞外基质组织是两个治疗组中最下调的反应组途径基因,这表明过度细胞外基质的产生降低(图。4e,f)。我们询问了使用我们的AI驱动目标发现平台Pandaomics,询问了多种发表的基因表达数据集,分析了IPF患者的组织以及健康个体的组织的组织37,对于这些顶级基因的表达模式以验证其与IPF的相关性。Col1a1,,,,MMP10和FAP发现与健康个体相比,IPF患者样本中普遍上调FN1
在大多数数据集中被上调(扩展数据图。7a)。我们进一步发现,以前报道的蛋白质的第12周的丰度与肺功能有关38和无移植生存39在IPF患者中,例如ADAMTSL2,COL6A3,CCN3,COL24A1,KRT19和LTBP2患者,是FVC的相关变化(扩展数据图。7b)。发现与炎症相关途径的表达,其他免疫相关蛋白(例如IL-10和CD5)以及其他细胞外基质相关蛋白(例如COL6A3)的表达,与FVC的治疗和变化具有显着关联,进一步表明TNIK在各种老化的途径中发挥了多样的途径,36(如图。
4e,f并扩展数据图。8)。讨论在这项2A阶段的研究中,在30毫克QD,30毫克竞标和60毫克QD的Rentosertib在12周内进行的治疗是安全且耐受性良好的。与治疗相关的SAE很少见,在治疗和安慰剂组中的率也很低。导致终止治疗的最常见茶是肝毒性相关的,主要发生在也接受过耐糖抗抗纤维化疗法的患者中,尽管尤其不是吡啶酮。除肝毒性外,腹泻和低钾血症是接受伦瑟替伯治疗的参与者中最常见的茶点之一。为了确定Rentosertib和SOC抗纤维化疗法对肝脏和胃肠道毒性的各自和协同贡献,以及对吸收或谷水平的任何潜在影响,我们需要进一步的药物相互作用研究,并评估较大的混合SOC抗抗纤维化的受体和非发病率的同类群。在12周内用60毫克Rentosertib QD进行治疗与IPF患者FVC增加的趋势有关。60毫克的Rentosertib QD剂量表现出肺功能的平均改善,如FVC所测量,而接受安慰剂的患者的平均FVC平均下降。接受60毫克Rentosertib QD的患者通过我们的建模而平均增加了FVC百分比变化2.82%,达到FVC在IPF中报告的最小临床重要差异(MCID)为2 h 6%(参考。40)。
通过同时进行SOC抗纤维化治疗的亚组患者表明,患者接受60毫克的Rentosertib QD而没有SOC抗抗纤维化的患者表现出FVC的最大增加,这表明这些药物之间的潜在相互作用将在以后的试验中进行研究。
FVC与稳定DLCO的不一致可能归因于每个治疗组中患者数量少,并且DLCO测量的固有变异性
41。Rentosertib治疗对QOL指标的影响在很大程度上尚无定论,但在治疗组中的差异很大,尽管我们的模型发现患者报告的与咳嗽相关的QOL(通过LCQ)在接受60毫克Rentosertib QD的患者中得到了显着改善。由于各种QOL指标整合了许多器官系统的功能,其中肺部健康是一个促成因素,因此治疗组之间缺乏差异可能是由于数据收集期相对较短,同队的大小较小。AE-IPF是患者预后惨淡的强烈迹象42
发病后2.2个月的中位生存期
43并且发病率符合长期治疗功效。在60毫克的Rentosertib手臂中发生了三次急性加重,安慰剂臂中发生了三个急性加重。但是,一项为期12周的研究是捕获此类长期疾病事件或轨迹的相对较短的时间范围44。Rentosertib潜在的免疫调节作用机理的意外后果9可能是一种缩减的部位特异性免疫反应,可能导致感染的敏感性增加,这是AE-IPF子集的已知触发因素45,,,,46。由于AE-IPF是临床上重要的事件,因此我们打算在将来具有较大人群的更长试验中评估其发病率,该试验将至少评估患者至少12个月,以更充分地捕获AE-IPF和其他潜在AE的发生率。在患者中测定血清蛋白质组,可洞悉与较高剂量rentosertib相关的循环蛋白质变化。
顶部和下调的蛋白质在30毫克竞标和60毫克QD剂量之间显示出很高的重叠,其下调的蛋白质富含与细胞外基质组织的关联,这是IPF纤维化进展的关键特征。此外,已知许多顶部下调的蛋白质与肺纤维化有关:MMP1047,LTBP239,,,,48,,,,49,KRT1939和COL24A139已被提名为IPF和无移植生存的生物标志物;ptprz150激活α2-catenin信号传导,这有助于IPF开发51,BHLHE40通过IPF中的上皮到间质转变,通过²-catenin52;COL1A1是纤维化的细胞外基质沉积特征失调的关键组成部分53,,,,54,,,,55;ASPN在肌纤维细胞转换中起作用56并且可能在IPF组织重塑中起着核心作用55,,,,57;循环ROBO2与IPF预后不良有关58;FAP在IPF患者的肺中仅在纤维化基因座中表达59;乍得与软骨纤维化基因座中的II型胶原蛋白结合60,,,,61。这种与IPF相关的蛋白质谱的下调支持以下假设:抑制TNIK可调节IPF的病理生理途径,并指出潜在的血清蛋白质特征作为对Rentosertib治疗的反应的生物标志物。
这项研究的局限性包括每个手臂的小队列大小,参与者的地理和人口统计学同质性(所有人都是类似种族的居民)和短期的随访,这限制了对长期安全性和有效性的评估。尽管持续时间很短,并且从所有武器中审判的撤离数量(n= 16/71(22.5%)),令人鼓舞的结果是对该药物候选者和靶标进行进一步研究,这表明Rentosertib通常是安全且可能有效的,并且更广泛地,在靶标识别和药物设计中使用AI可以提高药物开发过程的效率。必须在全球范围内的IPF患者中研究Rentosertib的更长的第2或3阶段试验,以进一步评估Rentosertib治疗IPF的疗效和安全性。
方法
研究设计
This phase 2a, multicenter, double-blind, placebo-controlled, randomized, multidose trial of rentosertib in adults with IPF was performed at 21 sites across China beginning 19 July 2023 and running through 11 June 2024. Adults with IPF were randomly assigned in a 1:1:1:1 ratio via interactive response technology to receive oral rentosertib (at a dose of 30 mg (QD), 30 mg(BID)或60毫克(QD))或安慰剂(QD)持续12周,并继续使用SOC药物(图。1)。在第二次访问时第一次服用前30天,患者筛查平均进行。患者在第1天,第2周,第4周,第8周,第12周(治疗结束)和第13周(研究结束)对患者进行临床评估。研究治疗Rentosertib由Insilico Medicine或指定的合同研究组织提供,并根据良好的制造实践的原理包装和标记。通过交互式响应技术系统对网站进行补给,该系统还监视了该站点可用的供应日期。控制并记录了对随机代码的访问。
该试验是根据赫尔辛基宣言和国际良好临床实践统一指南宣言中概述的原则进行的。参与中心的机构审查委员会或道德委员会批准了协议,并在启动临床试验之前遵守当地法律。本研究中的所有患者均提供了书面知情同意。试验管理和数据处理,汇总和分析是由英国利兹的Fortrea Clinical Phartical药理学服务进行的。
研究赞助商使用的作者参与了试验设计,收集,分析和数据解释。受试者,研究人员,现场研究人员,审阅者以及参与研究行为或分析的每个人都对随机治疗分配造成了视而不见的,直到数据冻结后。允许生物分析人员从分配给安慰剂治疗的受试者中识别样本,但在审判之前没有披露随机分组。这些作者在手稿的所有迭代中为手稿准备做出了贡献。这项研究的第一位也是最后一位作者保证了本出版物中报告的所有数据的完整性。
参与者
符合条件的患者为40岁或以上的IPF诊断,由美国胸部学会,欧洲呼吸学会,日本呼吸学会和拉丁美洲胸腔协会指南定义62。注册的患者呈现稳定的IPF,被认为适合根据病史,体格检查,生命体征,12铅ECG和实验室评估进行研究。如果先前接受Nintedanib或Pirfenidone的患者在第一次(筛查)访问前稳定了8周,则会招募耐纤维化疗法。符合条件的患者必须在筛查期间符合以下所有三个标准:(1)FVC> 40%的正常预测,(2)DLCO校正了血红蛋白的25%和<80%的正常预测,(3)FEV1/FVC比率> 0.7,基于PreChonChodiLator估算。患者因伴随呼吸系统疾病或健康问题而被排除在外,包括但不限于囊性纤维化,活跃的曲霉菌病,活性结核病,证实了2019年的冠状病毒病2019(COVID-19)(COVID-19)(COVID-19)(COVID-19)访问1或2或严重的COVID,需要在6个月内进行一次访问1或长时间的诊所诊断诊所,临床诊所,或者需要住院。在访问1和/或筛查期间的4个月内,在访问前的4周内和/或访问前的4周内和/或访问2(第1天)在肺部移植登记册上有4个月内尚未完全解决,在肺移植登记册上,或预计将在6个月内活跃于6个月前的肺部或预期在肺部转移1月4日,肺部的历史,肺部的历史,肺部的历史,肺部的历史,肺部的历史,肺部的较量恢复量很大,在肺部移植量上>2,天冬氨酸氨基转移酶(AST)或Alt - 正常或总胆红素的上层1.5倍 - 拜访1的正常上层1.5倍1.5倍,egfr - 60 ml” min11.73 m2(慢性肾脏疾病流行病学协作公式)在访问1时,不受控制的高血压(收缩压> 160 -mmHg或舒张压> 95毫米> 95毫米,在访问1中进行治疗1),不稳定的心脏和心脏梗死或在1个月内访问1个月,在1个月内访问1个月,在1个月内进行了任何证明或病历。 malignancy within 5 years before visit 1, known hypersensitivity or contraindications to serine/threonine kinase inhibitors, any condition or treatment possibly affecting drug absorption (for example, gastrectomy or metoclopramide), taking restricted medications (for example, moderate-to-strong CYP3A4–CYP1A2 inhibitors or inducers or medications primarily metabolized by CYP3A4–CYP1A2) or consumption of grapefruit or grapefruit juice, pomelo, Seville orange or Seville orange-containing products within 48 h before day 1, substantial trauma or major surgery within 3 months before visit 1 or planned major surgery during the study and 12-lead ECG demonstrating corrected QT interval by Fridericia (QTcF) >450 ms for males and >470 ms for females, or a QRS interval >120 ms at visit 1. Full inclusion and exclusion criteria are detailed in the associated study protocol.
Given an approximate sample size of 15 subjects per treatment arm, there exists a 90% probability of observing at least one AE if the true population rate is approximately 15%, which is sufficient to assess the feasibility of safety parameters.
Objectives and endpoints
The primary objective of this study was to evaluate the safety and tolerability of orally administered rentosertib for up to 12 weeks in adult patients with IPF compared with placebo.The primary endpoint for this study was the percentage of patients who have at least one TEAE.Patients were continually monitored for AEs during the 12 weeks of rentosertib treatment and for 1 week after administration of the final dose, with study drug-related SAE and AEs of special interest collected if they occurred beyond the study period.
The secondary efficacy endpoints measured the relative and percent change in FVC from week 0/visit 2 up to week 12. The absolute and relative change in FVC (%) predicted from week 0/visit 2 up to week 12 as well as the change in DLCO (%) predicted from week 0 up to week 12 was also measured.Twenty-one subjects (29.6%) had FVC and FEV1 measured at baseline (randomization with spirometry equipment sourced from the local site meeting the 2019 American Thoracic Society and European Respiratory Society guideline criteria)63, provided that the subject’s baseline and end-of-treatment spirometry were performed with the same equipment.Fifty subjects (70.4%) had FVC and FEV1 measured at baseline/randomization with centrally sourced SpiroSphere devices (Clario) provided by the sponsor with study-specific training and proficiency tests to the operating staff to meet the 2019 ATS/ERS guideline criteria63with central overread.All DLCO measurements were assessed with devices from each site according to the ATS/ERS guidelines64, and all measurements were conducted with the same equipment for each site.FVC MCID has been reported to be a change of 2–6% in patients with IPF40。
The change in LCQ, a 19-item questionnaire that assesses cough-related QOL65, from week 0 to weeks 4, 8 and 12 was also analyzed.The LCQ examines three domains (physical, psychological and social) related to the patient’s experience within a 24-h time frame.The range for the total score on the LCQ is 3–21, with the sum of each domain score ranging from 1 to 7. A higher score indicates a better QOL, with an MCID reported to be a change of 1.3 points for patients with chronic obstructive pulmonary disease66。The LCQ questionnaire has mainly been validated in disease settings such as chronic obstructive pulmonary disease67, noncystic fibrosis bronchiectasis68and chronic cough69。
还评估了从第0周增加到第12周6MWD的变化。
其他次要终点包括Rentosertib的药代动力学参数和相关代谢物在第2次访问2和访问6(EOT)的最后剂量之后。这项研究还收集了有关急性IPF加重的数量的数据,以及从第0周到第12周,急性IPF加重后住院的天数。
The exploratory endpoints analyzed include the change in IPF blood biomarkers after rentosertib treatment at weeks 0, 2, 4, 8 and 12. Changes in the blood proteome in treated patients were also measured at weeks 0, 2, 4, 8 and 12.
统计分析
An analysis of covariance (ANCOVA) model was performed for endpoints including FVC.The model includes treatment as a fixed effect and baseline value as a covariate.LCQ scores were analyzed using a mixed model for repeated measures.FVC and other spirometry measurements were collected at baseline and at week 12, with all patients being measured at baseline and a total of 22 patients missing week 12 measurements (5 receiving 30 mg rentosertib QD, 6 receiving 30 mg rentosertib BID, 8 receiving 60 mg rentosertib QD and 3 receiving placebo).Missing values at week 12 were imputed using a multiple imputation method assuming missing at random.All statistical comparisons were made using two-sided tests.No statistical comparisons of AEs between treatment groups were performed.Given an approximate sample size of 15 subjects per treatment arm, there exists a 90% probability of observing at least one AE if the true population rate is approximately 15%, which was sufficient to assess the feasibility of safety parameters, although a sample size calculation based on statistical power considerations was not performed.Spearman correlation analysis was used to assess the association between FVC change and AUC0–t或者c槽。
The safety population included all subjects who received at least one dose of study treatment.The intent-to-treat population included any randomized subjects.The intent-to-treat population was used for summary of demographic and baseline characteristics and for analysis of secondary endpoints, except PK analysis.Assessment of PK parameters included subjects who received at least one dose of study treatment and had at least one measurable concentration collected after dosing.Assessment of serum biomarkers was performed on subjects who had a predose baseline value and at least one value after treatment initiated.
For Olink proteomics analyses, we utilized a generalized linear mixed model to assess the association between each protein and dose or FVC change.The formula for modeling the treatment effect is
$${{\mathrm{NPX}}}\left({{\mathrm{protein}}\; {\mathrm{level}}}\right) \approx {{\mathrm{treatment}}}+{{\mathrm{visit}}}+{{\mathrm{treatment}}}:{{\mathrm{visit}}}+\left(1|{{\mathrm{PatientID}}}\right).$$
The lme4 package was used for the generalized linear mixed model analysis.For modeling the FVC changes, we conducted the statistical analyses in two steps.In the first step, we regressed the protein level (NPX) against the visit to obtain the slope of protein change rate for each individual.In the second step, we regressed the FVC changes against the slope of protein change rates obtained from the first step.This was conducted using the lm package.Pathway analyses were performed using the clusterProfiler R package, with Reactome processes70as the curated pathway set.
报告摘要
Further information on research design is available in theNature Portfolio Reporting Summarylinked to this article.
数据可用性
Complete deidentified Olink proteomics data, and FVC data, have been deposited at the OMIX database under accession codes at OMIX008341 (https://ngdc.cncb.ac.cn/omix/release/OMIX008341)。Qualified researchers may request access to Olink proteomics data through the OMIX database athttps://ngdc.cncb.ac.cn/omix/。Study protocol and statistical analysis plan will be provided in a secure data sharing environment upon academic or research request.源数据are provided with this paper.
代码可用性
Custom code used to analyze Olink proteomics data is available via GitHub athttps://github.com/HUICUI1992/Code-for-NM。参考
Scannell, J. W., Blanckley, A., Boldon, H. & Warrington, B. Diagnosing the decline in pharmaceutical R&D efficiency.
纳特。Drug Discov牧师。 11, 191–200 (2012).
文章一个 CAS一个 PubMed一个 Google Scholar一个
Berdigaliyev, N. & Aljofan, M. An overview of drug discovery and development.Future Med Chem。 12, 939–947 (2020).
文章一个 CAS一个 PubMed一个 Google Scholar一个
Pun, F. W., Ozerov, I. V. & Zhavoronkov, A. AI-powered therapeutic target discovery.趋势Pharmacol。科学。 44, 561–572 (2023).
文章一个 CAS一个 PubMed一个 Google Scholar一个
Qureshi, R. et al.药物发现中的AI及其临床相关性。Heliyon 9, e17575 (2023).
文章一个 CAS一个 PubMed一个 PubMed Central一个 Google Scholar一个
You, Y. et al.Artificial intelligence in cancer target identification and drug discovery.Signal Transduct.目标。ther。 7, 156 (2022).
文章一个 PubMed一个 PubMed Central一个 Google Scholar一个
Du, Y. et al.Machine learning-aided generative molecular design.纳特。马赫。Intell。 6, 589–604 (2024).
文章一个 Google Scholar一个
Cheng, Y., Gong, Y., Liu, Y., Song, B. & Zou, Q. Molecular design in drug discovery: a comprehensive review of deep generative models.简短的。生物知识。 22, bbab344 (2021).
文章一个 PubMed一个 Google Scholar一个
Sousa, T., Correia, J., Pereira, V. & Rocha, M. Generative deep learning for targeted compound design.J. Chem。Inf.模型。 61, 5343–5361 (2021).
文章一个 CAS一个 PubMed一个 Google Scholar一个
Ren, F. et al.A small-molecule TNIK inhibitor targets fibrosis in preclinical and clinical models.纳特。生物技术。 https://doi.org/10.1038/s41587-024-02143-0(2024)。
Xu,J。等。Discovery of a novel and potent cyclin-dependent kinase 8/19 (CDK8/19) inhibitor for the treatment of cancer.J. Med。化学 67, 8161–8171 (2024).
文章一个 CAS一个 PubMed一个 Google Scholar一个
Aliper, A. et al.Prediction of clinical trials outcomes based on target choice and clinical trial design with multi-modal artificial intelligence.临床Pharmacol。ther。 114, 972–980 (2023).
文章一个 PubMed一个 Google Scholar一个
Feuerriegel, S. et al.因果机器学习,用于预测治疗结果。纳特。Med. 30, 958–968 (2024).
文章一个 CAS一个 PubMed一个 Google Scholar一个
Feijoo, F., Palopoli, M., Bernstein, J., Siddiqui, S. & Albright, T. E. Key indicators of phase transition for clinical trials through machine learning.毒品。今天 25, 414–421 (2020).
文章一个 PubMed一个 Google Scholar一个
Artemov, A. V. et al.Integrated deep learned transcriptomic and structure-based predictor of clinical trials outcomes.Preprint atBiorxiv https://doi.org/10.1101/095653(2016)。
Gayvert, K. M., Madhukar, N. S. & Elemento, O. A data-driven approach to predicting successes and failures of clinical trials.Cell Chem.生物。 23, 1294–1301 (2016).
文章一个 CAS一个 PubMed一个 PubMed Central一个 Google Scholar一个
Lavecchia, A. Navigating the frontier of drug-like chemical space with cutting-edge generative AI models.毒品。今天 29, 104133 (2024).
文章一个 CAS一个 PubMed一个 Google Scholar一个
Jiménez-Luna, J., Grisoni, F., Weskamp, N. & Schneider, G. Artificial intelligence in drug discovery: recent advances and future perspectives.Expert Opin.毒品。 16, 949–959 (2021).
文章一个 PubMed一个 Google Scholar一个
Mak, K.-K.& Pichika, M. R. Artificial intelligence in drug development: present status and future prospects.毒品。今天 24, 773–780 (2019).
文章一个 PubMed一个 Google Scholar一个
Jayatunga, M. K. P., Xie, W., Ruder, L., Schulze, U. & Meier, C. AI in small-molecule drug discovery: a coming wave?纳特。Drug Discov牧师。 21, 175–176 (2022).
文章一个 CAS一个 PubMed一个 Google Scholar一个
Wong, C. H., Siah, K. W. & Lo, A. W. Estimation of clinical trial success rates and related parameters.生物统计学 20, 273–286 (2019).
文章一个 PubMed一个 Google Scholar一个
KP Jayatunga, M., Ayers, M., Bruens, L., Jayanth, D. & Meier, C. How successful are AI-discovered drugs in clinical trials?A first analysis and emerging lessons.毒品。今天 29, 104009 (2024).
文章一个 CAS一个 PubMed一个 Google Scholar一个
Pun, F. W. et al.Identification of therapeutic targets for amyotrophic lateral sclerosis using PandaOmics—an AI-enabled biological target discovery platform.正面。Aging Neurosci. 14, 914017 (2022).
文章一个 CAS一个 PubMed一个 PubMed Central一个 Google Scholar一个
Pun, F. W. et al.Hallmarks of aging-based dual-purpose disease and age-associated targets predicted using PandaOmics AI-powered discovery engine.老化 14, 2475–2506 (2022).
文章一个 CAS一个 PubMed一个 PubMed Central一个 Google Scholar一个
Raghu, G. et al.Diagnosis of idiopathic pulmonary fibrosis.An official ATS/ERS/JRS/ALAT clinical practice guideline.是。J. Respir。暴击。护理医学。 198, e44–e68 (2018).
文章一个 PubMed一个 Google Scholar一个
Lederer, D. J. & Martinez, F. J. Idiopathic pulmonary fibrosis.N. Engl。J. Med。 378, 1811–1823 (2018).
文章一个 CAS一个 PubMed一个 Google Scholar一个
Richeldi, L., Collard, H. R. & Jones, M. G. Idiopathic pulmonary fibrosis.柳叶刀 389, 1941–1952 (2017).
文章一个 PubMed一个 Google Scholar一个
Raghu, G. & Richeldi, L. Current approaches to the management of idiopathic pulmonary fibrosis.呼吸。Med. 129, 24–30 (2017).
文章一个 PubMed一个 Google Scholar一个
Martinez, F. J. et al.Idiopathic pulmonary fibrosis.纳特。修订版。Prim. 3, 17074 (2017).
文章一个 PubMed一个 Google Scholar一个
Raghu, G. Idiopathic pulmonary fibrosis: lessons from clinical trials over the past 25 years.欧元。呼吸。J. 50, 1701209 (2017).
文章一个 PubMed一个 Google Scholar一个
King, T. E., Tooze, J. A., Schwarz, M. I., Brown, K. R. & Cherniack, R. M. Predicting survival in idiopathic pulmonary fibrosis.是。J. Respir。暴击。护理医学。 164, 1171–1181 (2001).
文章一个 PubMed一个 Google Scholar一个
Wollin, L. et al.Nintedanib在特发性肺纤维化治疗方面的作用方式。欧元。呼吸。J. 45, 1434–1445 (2015).
文章一个 CAS一个 PubMed一个 PubMed Central一个 Google Scholar一个
Aimo, A. et al.Pirfenidone for idiopathic pulmonary fibrosis and beyond.卡片。Fail Rev. 8, e12 (2022).
文章一个 PubMed一个 PubMed Central一个 Google Scholar一个
Noble, P. W. et al.Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials.柳叶刀 377, 1760–1769 (2011).
文章一个 CAS一个 PubMed一个 Google Scholar一个
Richeldi, L. et al.Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis.N. Engl。J. Med。 370, 2071–2082 (2014).
文章一个 PubMed一个 Google Scholar一个
King, T. E. et al.吡非酮对特发性肺纤维化患者的3期试验。N. Engl。J. Med。 370, 2083–2092 (2014).
文章一个 PubMed一个 Google Scholar一个
Ewald, C. Y. et al.TNIK’s emerging role in cancer, metabolism, and age-related diseases.趋势Pharmacol。科学。 45, 478–489 (2024).
文章一个 CAS一个 PubMed一个 Google Scholar一个
Kamya, P. et al.PandaOmics: an AI-driven platform for therapeutic target and biomarker discovery.J. Chem。Inf.模型。 64, 3961–3969 (2024).
文章一个 CAS一个 PubMed一个 PubMed Central一个 Google Scholar一个
Su, C.-Y.等。Multi-ancestry proteome-phenome-wide Mendelian randomization offers a comprehensive protein-disease atlas and potential therapeutic targets.Preprint atmedrxiv https://doi.org/10.1101/2024.10.17.24315553(2024)。
Oldham, J. M. et al.Proteomic biomarkers of survival in idiopathic pulmonary fibrosis.是。J. Respir。暴击。护理医学。 209, 1111–1120 (2024).
文章一个 CAS一个 PubMed一个 Google Scholar一个
du Bois, R. M. et al.Forced vital capacity in patients with idiopathic pulmonary fibrosis: test properties and minimal clinically important difference.是。J. Respir。暴击。护理医学。 184, 1382–1389 (2011).
文章一个 PubMed一个 Google Scholar一个
McCormack, M. C. Facing the noise: addressing the endemic variability in DLCO testing.呼吸。关心 57, 17–25 (2012).
文章一个 PubMed一个 Google Scholar一个
Collard, H. R. et al.特发性肺纤维化的急性加重。是。J. Respir。暴击。护理医学。 176, 636–643 (2007).
文章一个 PubMed一个 Google Scholar一个
Song, J. W., Hong, S.-B., Lim, C.-M., Koh, Y. & Kim, D. S. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome.欧元。呼吸。J. 37, 356–363 (2011).
文章一个 CAS一个 PubMed一个 Google Scholar一个
Richeldi, L. et al.对特发性肺纤维化的优先磷酸二酯酶4B抑制剂的试验。N. Engl。J. Med。 386, 2178–2187 (2022).
文章一个 CAS一个 PubMed一个 Google Scholar一个
Wootton, S. C. et al.特发性肺纤维化的急性加重病毒感染。是。J. Respir。暴击。护理医学。 183, 1698–1702 (2011).
文章一个 PubMed一个 PubMed Central一个 Google Scholar一个
Collard, H. R. et al.Acute exacerbation of idiopathic pulmonary fibrosis.An International Working Group Report.是。J. Respir。暴击。护理医学。 194, 265–275 (2016).
文章一个 CAS一个 PubMed一个 Google Scholar一个
Sokai, A. et al.Matrix metalloproteinase-10: a novel biomarker for idiopathic pulmonary fibrosis.呼吸。res。 16, 120 (2015).
文章一个 PubMed一个 PubMed Central一个 Google Scholar一个
Enomoto, Y. et al.LTBP2 is secreted from lung myofibroblasts and is a potential biomarker for idiopathic pulmonary fibrosis.临床科学。 132, 1565–1580 (2018).
文章一个 CAS一个 Google Scholar一个
Zou, M. et al.Plasma LTBP2 as a potential biomarker in differential diagnosis of connective tissue disease-associated interstitial lung disease and idiopathic pulmonary fibrosis: a pilot study.临床经验。Med. 23, 4809–4816 (2023).
文章一个 CAS一个 PubMed一个 Google Scholar一个
Shang, D., Xu, X., Wang, D., Li, Y. & Liu, Y. Protein tyrosine phosphatase ζ enhances proliferation by increasing β-catenin nuclear expression in VHL-inactive human renal cell carcinoma cells.World J. Urol. 31, 1547–1554 (2013).
文章一个 CAS一个 PubMed一个 Google Scholar一个
Chilosi, M. et al.Aberrant Wnt/β-catenin pathway activation in idiopathic pulmonary fibrosis.是。J. Pathol。 162, 1495–1502 (2003).
文章一个 CAS一个 PubMed一个 PubMed Central一个 Google Scholar一个
Hu, X. et al.Dec1 deficiency ameliorates pulmonary fibrosis through the PI3K/AKT/GSK-3β/β-catenin integrated signaling pathway.正面。Pharmacol。 13, 829673 (2022).
文章一个 CAS一个 PubMed一个 PubMed Central一个 Google Scholar一个
Devos, H., Zoidakis, J., Roubelakis, M. G., Latosinska, A. & Vlahou, A. Reviewing the regulators of COL1A1.int。J. Mol。科学。 24, 10004 (2023).
文章一个 CAS一个 PubMed一个 PubMed Central一个 Google Scholar一个
Bibaki, E. et al.miR-185和miR-29a在IPF和肺癌的支气管肺泡细胞中类似地表达,但共同的靶标DNMT1和COL1A1显示出疾病特定的模式。摩尔。Med.代表。 17, 7105–7112 (2018).
CAS一个 PubMed一个 PubMed Central一个 Google Scholar一个
Wan, H. et al.通过生物信息学分析鉴定与特发性肺纤维化相关的枢纽基因和途径。正面。摩尔。Biosci。 8, 711239 (2021).
文章一个 CAS一个 PubMed一个 PubMed Central一个 Google Scholar一个
Huang,S。等。Asporin promotes TGF-β–induced lung myofibroblast differentiation by facilitating Rab11-dependent recycling of TβRI.是。J. Respir。细胞分子。生物。 66, 158–170 (2022).
文章一个 CAS一个 PubMed一个 Google Scholar一个
Ã…hrman, E. et al.慢性阻塞性肺疾病和特发性肺纤维化中肺细胞外基质的定量蛋白质组学表征。J. Proteom。 189, 23–33 (2018).
文章一个 Google Scholar一个
Todd, J. L. et al.Association of circulating proteins with death or lung transplant in patients with idiopathic pulmonary fibrosis in the IPF-PRO Registry Cohort.肺 200, 11–18 (2022).
文章一个 CAS一个 PubMed一个 PubMed Central一个 Google Scholar一个
Acharya, P. S., Zukas, A., Chandan, V., Katzenstein, A.-L.A. & Puré, E. Fibroblast activation protein: a serine protease expressed at the remodeling interface in idiopathic pulmonary fibrosis.哼。Pathol。 37, 352–360 (2006).
文章一个 CAS一个 PubMed一个 Google Scholar一个
Hoffman, E. T. et al.Regional and disease specific human lung extracellular matrix composition.生物材料 293, 121960 (2023).
文章一个 CAS一个 PubMed一个 Google Scholar一个
Shum, L. Chondroadherin binds to type II collagen.关节炎。ther。 3, 72650 (2001).
文章一个 Google Scholar一个
Raghu, G. et al.Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline.是。J. Respir。暴击。护理医学。 205, e18–e47 (2022).
文章一个 PubMed一个 PubMed Central一个 Google Scholar一个
Graham, B. L. et al.Standardization of Spirometry 2019 Update.An Official American Thoracic Society and European Respiratory Society Technical Statement.是。J. Respir。暴击。护理医学。 200, e70–e88 (2019).
文章一个 PubMed一个 PubMed Central一个 Google Scholar一个
Graham, B. L. et al.2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung.欧元。呼吸。J. 49, 1600016 (2017).
文章一个 PubMed一个 Google Scholar一个
Birring, S. S. et al.Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ).胸部 https://doi.org/10.1136/thorax.58.4.339(2003)。
Rebelo, P. et al.Minimal clinically important differences for patient-reported outcome measures of cough and sputum in patients with COPD.Int J. Chron.阻碍。Pulmon.dis。 15, 201–212 (2020).
文章一个 PubMed一个 PubMed Central一个 Google Scholar一个
Berkhof, F. F. et al.The validity and precision of the Leicester Cough Questionnaire in COPD patients with chronic cough.Health Qual.Life Outcomes 10, 4 (2012).
文章一个 PubMed一个 PubMed Central一个 Google Scholar一个
Murray, M. P., Turnbull, K., MacQuarrie, S., Pentland, J. L. & Hill, A. T. Validation of the Leicester Cough Questionnaire in non-cystic fibrosis bronchiectasis.欧元。呼吸。J. 34, 125–131 (2009).
文章一个 CAS一个 PubMed一个 Google Scholar一个
Nguyen, A. M. et al.Leicester Cough Questionnaire validation and clinically important thresholds for change in refractory or unexplained chronic cough.ther。ADV。呼吸。dis。 16, 17534666221099737 (2022).
文章一个 PubMed一个 PubMed Central一个 Google Scholar一个
Milacic, M. et al.The Reactome Pathway Knowledgebase 2024.核酸res。 52, D672–D678 (2024).
文章一个 PubMed一个 Google Scholar一个
致谢
我们感谢Fortrea临床药理学服务(英国利兹)的后勤和组织支持,Insilico Medicine(中国上海,中国上海)的C. Wang and Y. Jiang of数据管理专业知识,Insilico Medicine的Y. Liu(上海,中国上海)的临床运营支持和Insilico Medicination(Cambridge for Cambridge fin Cambridge,MA)的临床支持。
道德声明
竞争利益
F.R., S.R., C.S., S.L., Y.L., H.Z., S.C., H.C., M.K., D.G.and A.Z.are employees of Insilico Medicine.Insilico Medicine was the study sponsor.其他作者没有宣称没有竞争利益。
同行评审
同行评审信息
自然医学thanks Yuben Moodley, Luca Richeldi, Jeff Swigris and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.Primary Handling Editor: Lorenzo Righetto, in collaboration with the自然医学团队。
附加信息
出版商的注释关于已发表的地图和机构隶属关系中的管辖权主张,Springer自然仍然是中立的。
扩展数据
Extended Data Fig. 1 Key trial eligibility criteria, randomization scheme, and schedule of key study activities.
Eligible patients were randomized to receive 30 mg rentosertib QD, 30 mg rentosertib BID, 60 mg rentosertib QD, or placebo administered over 12 weeks with periodic assessment and plasma sampling.IPF,特发性肺纤维化;FVC, forced vital capacity;FEV1, forced expiratory volume in one second;DLCO, diffusion capacity of the lung for carbon monoxide;SOC, standard-of-care;QD, once-daily;BID, twice-daily;HRCT, high-resolution computed tomography;6MWD, six-minute walk distance;LCQ, Leicester cough questionnaire;PK, pharmacokinetics;EOT, end-of-trial;EOS, end-of-study.
Extended Data Fig. 2 Changes in FVC after 12 weeks of treatment with rentosertib.
a-b) Changes in forced vital capacity (FVC) ± 95% CI after 12 weeks of treatment compared to baseline excluding n = 1 patient from the placebo group and n = 1 patient from the rentosertib 30 mg QD group who exhibited >600 mL difference between screening and baseline FVC measurements, making uncertain the baseline FVC values in those patients.Absolute change in FVC ± 95% CI (一个) and absolute change in FVC ± 95% CI ANCOVA Model with Multiple Imputation assuming missing at random (MAR) (b)。((c) Absolute change in FVC (% Predicted) ± SE, ANCOVA Model with Multiple Imputation assuming MAR.((d) Percentage change in FVC ± SE, ANCOVA Model with Multiple Imputation assuming MAR.((e) Change in percent predicted FVC ± SD.Extended Data Fig. 3 Changes in FVC after 12 weeks of treatment with rentosertib stratified by concurrent use of SOC antifibrotic therapy.
((一个
) Absolute change in FVC ± 95% CI in patients not concurrently taking SOC antifibrotic therapy (left) or in patients concurrently taking antifibrotic therapy (right).((b) Absolute change in FVC ± 95% CI by ANCOVA Model with Multiple Imputation assuming missing at random (MAR) in patients not concurrently taking SOC antifibrotic therapy (left) or in patients concurrently taking antifibrotic therapy (right).Extended Data Fig. 4 Changes in additional lung function metrics after 12 weeks of treatment with rentosertib.((一个
) Absolute change in DLCO ± 95% CI.
((b) Absolute Change in DLCO (% Predicted) ± SE, ANCOVA Model with Multiple Imputation assuming missing at random (MAR).((c) Absolute change in percent predicted HGB-corrected DLCO (%) ± SD.((d) Absolute change in FEV1 ± SD.((e) Mean percent-change in FEV1 ± SD.((f) Absolute change in LCQ score ± SD.((g) Absolute change in LCQ score ± SE by Mixed model for repeated measures (MMRM).((h) Absolute change in 6MWD ± SD.((我) Absolute change in 6MWD ± SE, ANCOVA Model with Multiple Imputation assuming MAR.Extended Data Fig. 5 Association of rentosertib treatment with PK parameters.((一个) AUC0-t
of rentosertib at week 0 and week 12, data are mean ± SD.
n = 11 at week 0 and n = 11 at week 12 in 30QD, n = 11 at week 0 and n = 10 at week 12 in 30 BID, n = 10 at week 0 and n = 10 at week 10 in 60 QD.((b)t1/2of rentosertib at week 0 and week 12, data are mean ± SD.n = 11 at week 0 and n = 11 at week 12 in 30QD, n = 11 at week 0 and n = 10 at week 12 in 30 BID, n = 10 at week 0 and n = 10 at week 10 in 60 QD.((c) Correlation analysis of AUC0-tand change in FVC between week 0 and week 12. (d) Correlation analysis of C槽measured throughout the trial and change in FVC between week 0 and week 12.pvalues calculated by two-sided Spearman correlation analysis.Extended Data Fig. 6 Time- and dose-associated changes in serum protein expression associated with rentosertib treatment.((一个) Change in Normalized Protein eXpression (NPX) from baseline to subsequent visits (weeks 4, 8, and 12) with a simple linear regression model (delta_NPX ~ treatment_cat) fitted to assess the relationship between treatment dose and protein expression changes using Benjamini-Hochberg (BH)-adjustedp值。
((b
) Change in NPX between baseline and week 12 by two-sided paired BH-adjusted t-test.Red dots denote proteins with BH-adjustedpvalue < 0.1.Yellow boxes highlight fibrosis-related proteins.Extended Data Fig. 7 Serum proteins with changes in expression associated with changes in FVC with rentosertib treatment.((一个) PandaOmics analysis of published RNA-seq gene expression datasets profiling patients with IPF and healthy individuals of four top proteomics hit genes in our trial cohort.((b
) Correlation analysis of protein abundance of genes previously associated with lung function and IPF TFS with change in FVC from week 0 to week 12, treatment regimen, and time on treatment.
n = 43 patient at each visit, and thepvalue was calculated by two-sided Pearson correlation analysis;n = 11 patients in Placebo, n = 11 patients in 30 QD;n = 11 patients in 30 BID, n = 10 patients in 60 QD.pvalues calculated by paired t-test.Extended Data Fig. 8 Correlation analysis of protein abundance of genes previously associated with aging-related dysfunction.Correlation analysis of protein abundance of genes (IL10, CD5, COL6A3) previously associated with aging-related dysfunction, such as immunity and ECM remodeling, with change in FVC from week 0 to week 12 and time on treatment.Left panel: n = 43 patient at each visit;
p
values calculated by two-sided Pearson correlation analysis;Right panel: n = 11 patients in placebo, n = 11 patients in 30 QD;n = 11 patients in 30 BID, n = 10 patients in 60 QD, boxes and whiskers are quartiles;pvalues calculated by paired t-test.补充信息
权利和权限
开放访问
本文在Creative Commons Attribution-Noncormercial-Noderivatives 4.0国际许可下获得许可,该许可允许任何非商业用途,共享,分发和复制以任何媒介或格式的形式,只要您提供适当的原始作者和来源的信用,请符合原始作者和来源,并提供了与Creative Commons的链接,并指示您是否修改了许可的材料。您没有根据本许可证的许可来共享本文或部分内容的改编材料。The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material.If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.要查看此许可证的副本,请访问http://creativecommons.org/licenses/by-nc-nd/4.0/。重印和权限
引用本文
Xu, Z., Ren, F., Wang, P.
等。A generative AI-discovered TNIK inhibitor for idiopathic pulmonary fibrosis: a randomized phase 2a trial.Nat Med(2025)。https://doi.org/10.1038/s41591-025-03743-2
已收到:
公认:
出版:
doi:https://doi.org/10.1038/s41591-025-03743-2