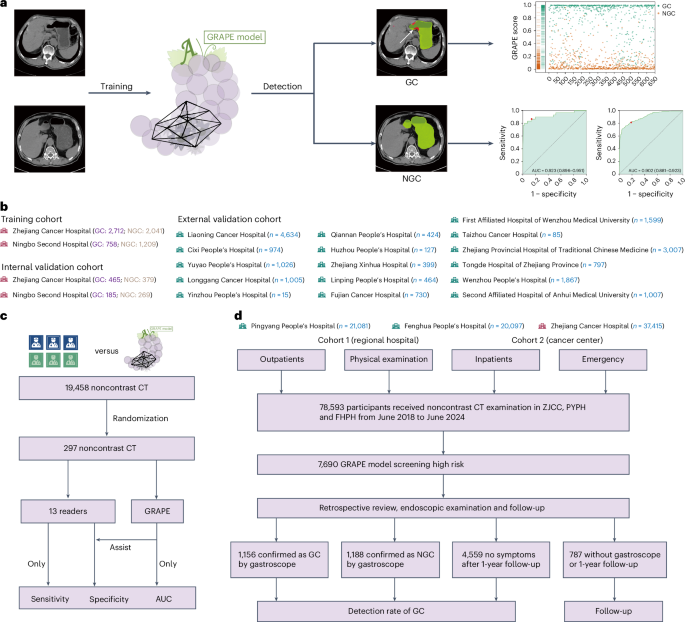
基于AI的非对比度CT成像对胃癌的大规模筛查
作者:Cheng, Xiangdong
胃癌(GC)是第五常见的癌症,是全球与癌症相关死亡的第四个主要原因
1,近75%的新病例和相关死亡发生在亚洲国家,尤其是中国,日本和韩国2,,,,3。有效筛查对于降低死亡率至关重要,因为研究表明,早期,可切除的GC(EGC)的5年生存率为95英寸99%,而晚期阶段(AGC)少于30%。4,,,,5。内窥镜检查是GC的标准诊断测试,因为它允许直接可视化胃粘膜和活检抽样以进行组织学评估。自1983年和1999年以来,日本和韩国分别通过内窥镜检查实施了国家GC筛查计划,导致早期诊断率更高和死亡率降低,与中国相比,日本(60.3%)和韩国(68.9%)的生存率明显高于中国(35.9%)(35.9%)6,,,,7。但是,在大多数GC患病率较高的国家中,通过内窥镜检查中总体中的大规模GC筛查是不可行的或具有成本效益的,导致死亡率很高。这主要是由于高成本,次优的检测率(被筛查总人数中检测到的GC病例的百分比)和内窥镜检查的侵入性性质,从而导致人群之间的依从性较低
8。筛查高危人是可取的,因为在阳性测试结果下,他们更有可能进行内窥镜检查。目前,血清学检查,包括幽门螺杆菌幽门螺杆菌血清学,血清胃蛋白原水平和胃蛋白17测试是最常用的方法,用于识别具有GC高风险的人。但是,血清学筛查和风险分层后通过胃镜检查的GC检测率仅为1.25%,与一般人群的胃镜筛查的检测率相比,有限的改善(1.20%)9。因此,迫切需要开发新的非侵入性,低成本,高效和可靠的筛查技术,以识别GC高风险的人。这种技术可以优先考虑高风险人群的内窥镜检查,提高大规模筛查计划的成本效益,并最终降低与GC相关的死亡率10。
非对比度计算机断层扫描(CT)是体格检查中心和医院中的低成本且广泛使用的成像方案(尤其是在低资源区域)11,,,,12。低剂量的非对比度胸部CT已成为临床实践中肺结核监测的已建立诊断范式13,,,,14。通常指示非对比度的腹部CT快速诊断急性疾病,评估创伤性损伤并评估与对比剂的禁忌症患者,这是对腹部病理学初步临床评估的关键工具。人工智能(AI)的最新进展已显示出通过非对比CT进行癌症筛查的有希望的结果,特别是在检测胰腺癌方面15。目前,对比增强的CT用于评估GC16,包括浸润深度,淋巴结转移和远处转移。然而,鉴定主要的GC病变,特别是评估侵袭深度,通常受胃填充和胃肠道蠕动的影响。这些局限性阻碍了CT扫描在GC评估中的作用的探索。常规CT成像,尽管是机会性筛查的有价值的工具11,在检测GC方面面临重大挑战。常规CT成像在GC检测中的值,尤其是非对比度CT,具有通过AI进一步探索的潜力。
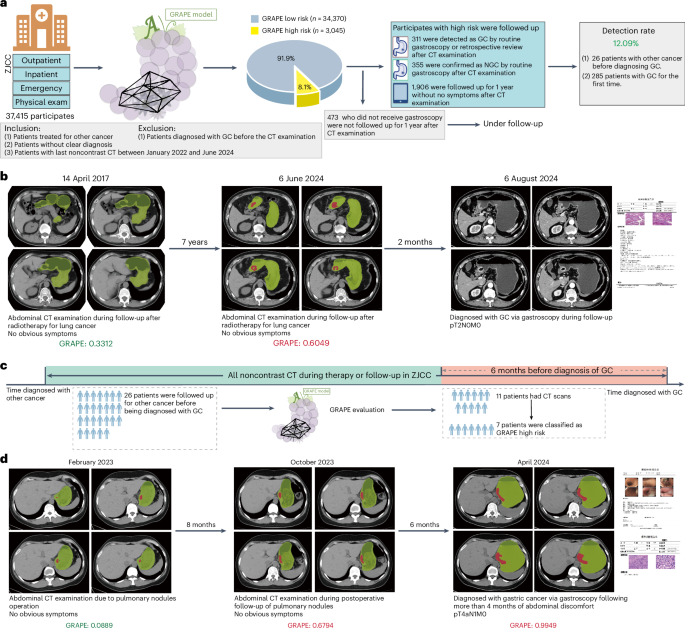
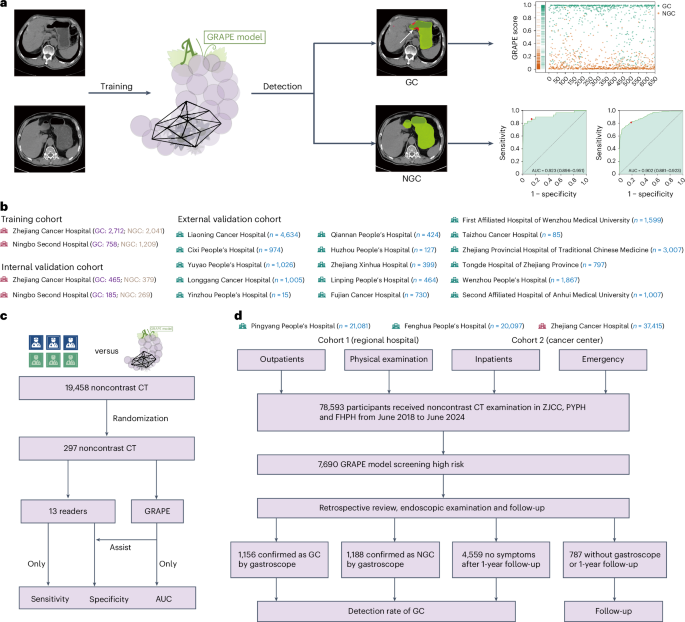
在这项研究中,我们提出了使用非对比度CT和深度学习来识别GC患者的葡萄(使用AI的GC风险评估程序)。该研究以三个不同的阶段进行。首先,我们使用来自中国两个中心的队列开发了葡萄,其中包括3,470例GC病例和3,250个非GC(NGC)病例。使用内部验证队列(1,298例)和从16个中心(18,160个案例)的独立外部验证队列验证了其有效性和效用。然后,我们将葡萄的解释与放射科医生的解释进行了比较,并探讨了其在解释过程中协助放射科医生的潜力。最后,我们使用两个现实世界的研究队列验证了葡萄在机会性筛查中的性能,其中包括78,593名参与者,其中一个来自一个综合癌症中心和两家独立的区域医院的非对比度CT。我们旨在验证第一个现实世界中的各种情况(身体检查,紧急情况,住院和门诊科)中包括在各种情况(身体检查,紧急情况,住院和门诊科中)以及对第二个现实世界中其他癌症进行治疗或随后在第二个现实世界中接受其他癌症的患者的常规患者的杂GC检测能力(图。1)。图1:葡萄的开发,评估和临床翻译概述。一个
结果
葡萄模型
葡萄模型是一个深度学习框架,旨在分析用于GC检测和分割的三维(3D)非对比度CT扫描。葡萄接受了内部培训队列的培训,包括3,470个GC病例和3,250个NGC案例(扩展数据表1)。它产生了两个输出:胃和肿瘤的像素级分割面膜,以及将GC患者与NGC区分开的分类评分。该模型遵循两阶段的方法(扩展数据图。1a)。在第一阶段,使用分割网络将胃定位在完整的CT扫描中,产生分割面罩,然后将其用于作物和分离胃部区域。这个裁剪的区域被送入第二阶段,其中采用了具有双分支的联合分类和分割网络。分割分支检测到已确定的胃部区域内的肿瘤,而分类分支集成了多级特征,以将患者分类为GC阳性或NGC。详细的描述,体系结构和葡萄的可解释性分析在方法并扩展数据图。1。
内部和外部评估
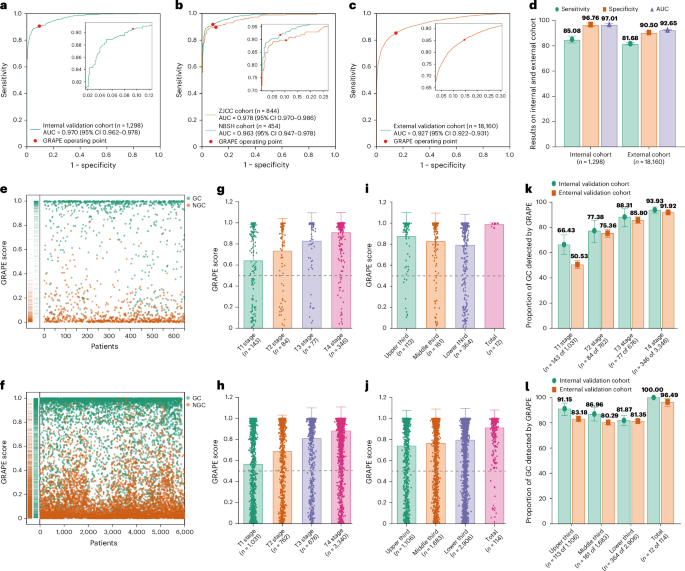
葡萄在接收器工作特性(ROC)曲线(AUC)下达到了0.970(95%置信区间(CI)0.962α0.978),敏感性为0.851(95%CI 0.825-0.877),特异性为0.968(95%CI0.953-0.953â0.980),在图中(图)2a,d),尽管外部验证确认了稳定的性能,AUC为0.927(95%CI 0.922-0.931),灵敏度为0.817(95%CI 0.807 0.827),特异性为0.905(95%CI 0.900 0.910)(图。2C,d和扩展数据表2)。千癌癌症中心(ZJCC)和宁波第二医院(NBSH)同伙之间没有统计学上的显着差异(图。2b和扩展数据表2)。多中心分析(n€€每100个中心)的AUC范围为0.902 0.995(扩展数据图2。2和3a和扩展数据表2)。图2:内部和外部验证队列中的葡萄性能。
,内部验证队列中的ROC葡萄曲线。b,内部验证队列中ZJCC和NBSH中葡萄的ROC曲线。c,外部验证队列中葡萄的ROC曲线。d内部和外部验证队列中的敏感性,特异性和葡萄的AUC。e,,,,f,内部GC和NGC葡萄得分的分布(e)和外部(f)验证队列。g,,,,h,内部不同t阶段的葡萄得分(g)和外部(h)验证队列。我,,,,j,内部不同位置的葡萄得分(我)和外部(j)验证队列。k,在内部和外部验证队列中不同t阶段在不同t阶段检测到的GC的比例。l,内部和外部验证队列中不同位置在胃的不同位置检测到的GC的比例。葡萄得分分布分析揭示了GC和NGC组之间的明显区别(图。2e,f和扩展数据表1),> 0.5分数在GC中占主导地位。T级进展显示出显着的得分升级(图。2G,h
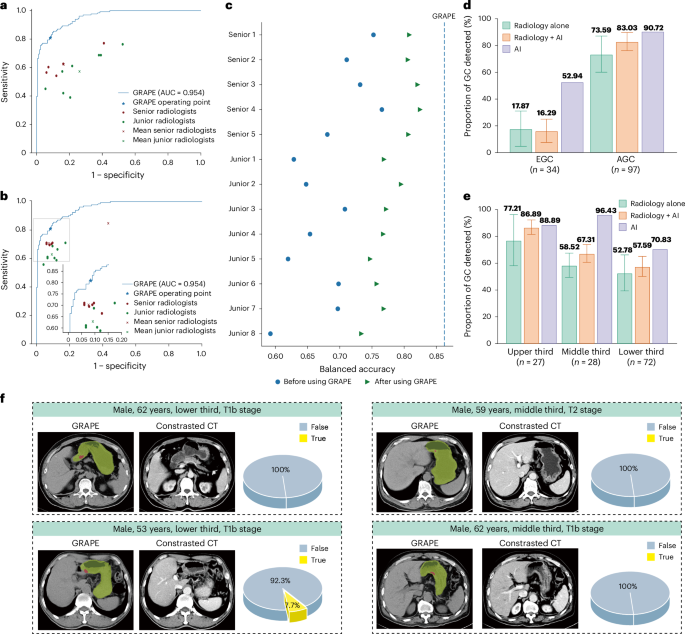
),而肿瘤位置没有相关性(图。2Gâj)。在两个队列中T阶段的进步随着T阶段的进步而增加。特定于位置的分析显示了全孔病变的最佳检测,在不同位置的性能相似(图。2k,l)。在相同的t阶段内,敏感性在整个位置保持一致(扩展数据图。3bâe)。值得注意的是,充实的胃显示EGC检测到10.72%的胃数比填充较差(扩展数据图。3f)。另外,GC的检测率与TNM阶段有关,但与性别或年龄没有相关性(扩展数据图。3g i)。读者研究在一项多中心读者研究中(n= 13个放射科医生)解释297个非对比度CT扫描,葡萄在诊断准确性方面超过了所有读者(AUC,葡萄0.92与读者相比,读取器范围为0.76 0.85),在敏感性和特异性方面表现出显着的性能优势。当在1个月的清除量后,通过AI辅助重新评估病例时,放射科医生的敏感性平均提高和特异性为13.3%。
值得注意的是,尽管高级和初级放射科医生都表现出明显的准确性提高(
p<0.05),它们的增强性能仍低于葡萄的独立结果(图。3aâc)。图3:读者研究。一个,葡萄与13位读者与13位放射科医生之间的比较,在GC上具有不同水平的专业知识。b
3e),这可能是由于难以在此位置识别出不同程度的胃填充的困难。数字3f表明放射科医生无法识别的T1/T2阶段GC的代表性CT扫描。医院机会筛查的现实世界研究我们使用两个现实世界中的研究队列进一步验证了机会性筛查中的葡萄性能,其中包括2018年至2024年之间连续78,593名参与者,来自两个独立的区域医院和一个综合癌症中心。
首先,我们在现实世界中验证了葡萄的机会性筛查能力,其中包括来自区域医院的41,178例常规患者(体格检查,紧急,住院和门诊部)。
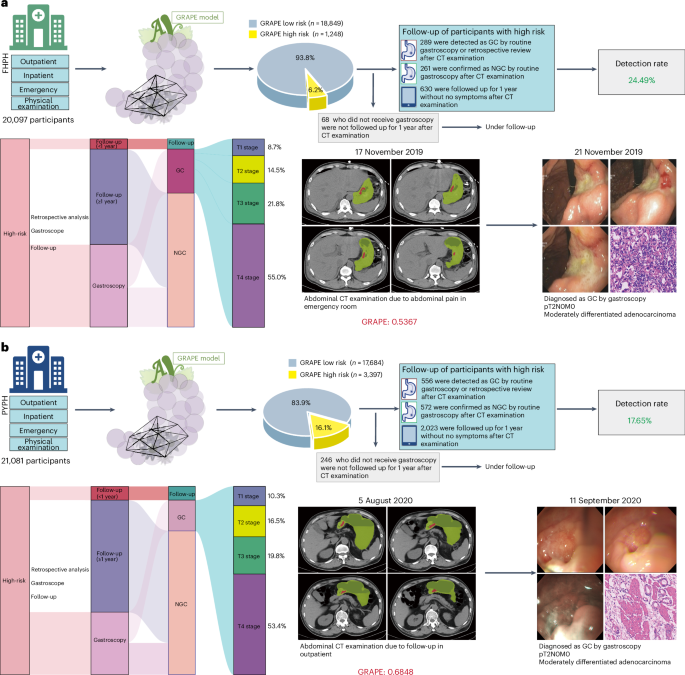
葡萄确定为11.28%(4,645)为高风险,在同类群体中有不同的比例:在FenghuaPeople(FHPH)的6.2%(20,097中的1,248个)医院(FHPH),而稀有Pinyang People(21,081的3,397)在Pinyang People)医院(pyph)(pyph)(pyph)(图)。4
)。通过全面的医疗记录审查和随访,我们将葡萄识别的高危患者分为四个临床轨迹:通过胃镜证实的GC,NGC诊断,无症状的1年随访患者以及缺乏胃镜和随访文档的患者。最后,在高危人群中,胃镜和随访验证显示,在FHPH中证实了289例GC病例,而Pyph中有556 GC(图。4a,b和扩展数据表4)。FHPH和PYPH的检测率分别为24.49%(1,180中的289个)和17.65%(3,151的556)。此外,我们回顾性地分析了高危人群中确认的GC患者,发现40.48%(289中的117)和38.31%(556个)患者分别在FHPH和PYPH中没有腹部症状。多学科团队(MDT)搜索并审查了FHPH和PYPH同类群中低风险患者的可用电子健康记录,包括所有现有的后续信息,并发现了37和49名GCS患者,并通过病理证实。因此,FHPH队列的估计灵敏度和特异性为0.887(95%CI 0.850 0.920)和0.951(95%CI 0.949 0.954),为0.919(95%CI 0.899 0.939)和0.861(95%CI 0.861)(95%CI 0.857.857 cohort cohort cohort con)
我们连续收集2022年1月至2024年6月在ZJCC之间的腹部非对比CT。葡萄确定8.1%(3,045)是高风险。全面的医学审查对证实了311例GC病例进行了分层(图。5a和扩展数据表4),包括34.41%(311例中的107)患者,没有明显的腹部症状。总检测率为12.1%。值得注意的是,一名患者在2024年6月在腹部非对比度CT扫描上被葡萄预测高风险。MDT审查显示该患者接受了胃镜检查,并于2024年8月被诊断为GC。手术病理学证实,GC为PT2N0M0(AJCC阶段IB)(AJCC IB)(AJCC IB)(AJCC IB)(图。5b)。
,在ZJCC队列中的葡萄性能概述。b,使用葡萄检测后诊断出的GC患者的例证。该患者于2024年6月被跟踪接受肺癌治疗,但被葡萄检测到。MDT审查显示,该患者在2024年8月被诊断为胃镜,并被诊断为中等分化的GC。c,CT扫描症状的数据收集过程。在其他癌症的随访中,有26例患者在GC诊断前的6个月内进行了诊断性CT扫描。葡萄在诊断前的6个月中,在63.64%(11名)患者中提出了GC。d,一名患者在诊断为GC前14个月和6个月的肺部结节诊断前接受了2次腹部CT检查,在2023年,没有明显的胃部异常。后来,该患者被诊断出在4月2024年4月2024年不得评估的腹腔镜中,通过胃镜进行了T4AN1M0阶段的GC诊断很差。诊断,结果表明,葡萄在诊断前6个月的非对比度CT中表明GC。基于对图像的葡萄预测和回顾性回顾,MDT怀疑该患者在T2期中,这是通过葡萄成功检测到的6个月。
我们验证了葡萄在早期诊断中的能力。在第二次现实世界中(ZJCC)中的311例GC病例中,有26例患者接受了随访,对其他癌症进行了审查或接受治疗,而其他285名患者在诊断为GC之前首次访问了医院。在26例患者中,有11例在GC诊断前的6个月内进行了腹部非对比度CT扫描。对11次诊断性扫描进行了测试,并预测了63.64%(11名)患者的GC(图。5C)。一名患者在诊断为肺结核引起的GC前14个月和6个月接受了两次腹部CT检查,在2023年没有明显的胃部异常。后来,该患者被诊断为在4月202日的腹部不及时诊断出4个月以上的4个月后,通过胃镜进行T4AN1M0阶段的GC诊断很差。结果表明,葡萄在诊断前6个月的非对比度CT中指示GC。基于对图像的葡萄预测和回顾性回顾,MDT怀疑该患者在T2阶段,这是通过葡萄成功检测到的6个月(图。5d)。
讨论
CT提供了对解剖结构的全面和客观评估,无论临床适应症如何17。AI方法的出现使通过深度学习,促进快速,客观算法的自动分割和处理未充分利用的数据,以进行准确的病变识别18,,,,19。在这项研究中,我们提出了基于常规的非对比度CT扫描进行GC检测的AI模型。在独立的外部多中心研究中,该模型的有效性得到了进一步验证。与基于临床信息和血清学诊断的先前模型相比,葡萄显示出GC的出色检测性能(AUC 0.757 0.79)20,,,,21,,,,22,性能与液体活检相当23,,,,24,包括圆形RNA25,microRNA26,,,,27,无细胞的DNA28和代谢物23在血液样本中,报告了AUC值在0.83至0.94之间。亚组分析表明,EGC的葡萄敏感性约为50%,T3和T4阶段的GC敏感性超过90%。尽管葡萄在晚期GC检测中表现出卓越的性能,但它可以在更早的阶段检测到GC,这也可以提高GC患者的总体存活率。
为了验证葡萄在现实的医院机会筛查中的价值,我们进行了两项连续参与者组成的实际研究。葡萄达到了高检测率,从基于问卷的方法中显着超过了所有GC的0.9%检测率29。葡萄在GC诊断前的6个月内进行的症状扫描也表现出良好的性能。尽管有优势,但葡萄并不打算替代内窥镜评估。我们认为,它为有症状的患者提供了一种有价值的替代方法,不愿接受初始内窥镜筛查。
目前,尽管已经针对其他恶性肿瘤进行了类似的研究,但在大规模筛选队列中尚未实施其他新的GC筛查方法。美国国家肺筛查试验报告了使用低剂量螺旋CT的肺癌检测率为2.4-5.2%30。使用经阴道超声检查的卵巢和输卵管癌的检测率为5.5%31。在液体筛查中,使用爱泼斯坦Barr病毒DNA水平在血浆样品中的鼻咽癌的检测率为11.0%32,通过无细胞的DNA检测在理论日食研究中,结直肠癌的检测率为3.2%33。作为一种高度准确的高风险识别工具,葡萄具有通过提高次级内窥镜检查的检测率来实现大规模筛查程序的潜力,从而降低了GC死亡率。
葡萄模型通过将分类和分割整合到单个深层学习框架中,采用简单而有效的架构来检测非对比度CT扫描的GC。传统方法通常仅专注于细分,从而限制了提供患者级别概率评估的能力,或者仅依靠直接应用于感兴趣的器官区域(ROI)的分类方法,从而降低了预测的可解释性。相比之下,我们的联合模型利用了两种策略的优势,使我们能够从详细的局部纹理模式识别肿瘤中受益,同时保持对胃的整体形状和结构的全面了解。通过改编NNUNET骨干,这是一种以其稳健和高性能的视觉特征提取能力而闻名的良好架构,我们的模型可以增强能力,以处理GC特有的医学图像中存在的复杂视觉特征。葡萄的整体设计不仅可以确保有效的功能学习,还可以改善各种数据集的概括。此外,就可解释性,葡萄的组合分割和分类管道而言,向临床医生提供了详细的图像,其中明确描绘了肿瘤分割掩模,同时提供决定性分类的输出。我们还通过GRAD-CAM进行了视觉分析的可解释性,发现粗热图与肿瘤区域很好。尽管非对比度CT不是GC诊断的常规方式,但可以通过葡萄的帮助来显着提高放射科医生的性能。这种改进可以归因于葡萄的肿瘤分割输出,这对于放射学家来说很容易解释。
尽管葡萄的结果令人鼓舞,但仍需要进一步的研究来解决其适用性的关键方面。首先,必须进行大量的前瞻性筛选队列,以更加稳健地验证GC筛查中的葡萄有效性。我们正在通过全国GC筛查计划实施对葡萄性能的大规模预期验证。由于场景差异,例如高风险与机会性筛查,预计将选择最佳的截止值以平衡临床实施过程中的灵敏度和特异性。其次,增强模型对早期GC的敏感性仍然是优先事项。我们将使用更多EGC扩大葡萄的训练数据集,从而提高模型敏感性。我们还将利用内窥镜和病理报告中的更多信息作为额外的监督,以训练葡萄模型,以进一步提高敏感性,例如形状,质地,大小,大小和侵袭肿瘤的程度。如前所述,胃填充更好的患者在早期GC检测中具有更高的敏感性。结果,我们建议在我们的前瞻性试验中进行非对比度CT成像之前的胃扩张。第三,尽管非GC肿瘤的患病率(例如胃肠道肿瘤和肠道相关的淋巴组织肿瘤)相对较低,但可以扩展葡萄以检测和诊断这些稀有肿瘤,从而使其成为更全面的筛查工具。最后,为了应对概括挑战,未来的方向将是从更多中心纳入培训数据。从技术方面,测试时间适应34Technique是一种最近的机器学习方法,可更好地建模通用性,并有可能提高葡萄的外部验证性能。总体而言,葡萄代表了一种大规模GC筛查的新方法,表明了高灵敏度,特异性和检测率,从而提高了GC筛查工作的成本效益和依从性。
方法
试用设计和人口
这项研究采用了相设计,包括三个独立的队列(图。1)。该研究得到了涵盖20个参与中心的集中机构审查委员会(IRB)(IRB-2024-279,ClinicalTrials.gov注册)批准NCT06614179)。培训队列在2006年9月至2024年6月之间在中国的2个中心招收,包括3,470名GC的参与者和3,250名NGC的参与者,年龄在18年99岁。内部验证队列在2006年12月至2024年4月之间在中国的2个中心招收,包括650名GC的参与者和648名NGC参与者,年龄在20年94岁。此外,在2011年1月至2024年8月之间,我们进行了独立的外部验证队列,招募了来自16个中心的18,160名参与者,他们接受了18岁的胃镜检查,年龄在80岁。GC组的纳入标准要求参与者通过胃镜病理活检确认对GC的诊断,并在诊断时接受任何治疗后进行了非对比度CT检查。如果参加者在接受治疗后接受了非对比度检查,则将被排除在外。对于NGC组,纳入标准要求参与者确认没有通过胃镜检查GC,并且在胃镜检查后的6个月内进行了非对比度CT检查。或者,如果NGC参与者进行了非对比度检查,并根据一年的随访未经GC进行确认。我们对所有入学的患者的病历进行了全面审查,包括年龄,性别,T级,位置等。内部和外部队列的完整详细信息,包括患者临床信息和CT获取参数,显示在扩展数据表中1。最后,我们使用2个现实世界的研究队列验证了现实的医院机会筛查中葡萄的性能,其中包括一个队列,其中包括2018年至2024年之间的41,178名参与者,由2家区域医院和一个同类群体组成,以及一个由37,415名参与者组成的37,415名参与者,与202222224和20224之间的参与者组成。总共有45.19%(20,097例中的9,083例)来自门诊和急诊科和体格检查中心,而54.80%(20,097例中的11,014例)患者来自FHPH的住院。
41.56%(21,081例中的8,761例)来自门诊和急诊科和体格检查中心,而58.44%(21,081例中有12,320)来自Pyph的住院。在ZJCC中,我们还排除了4,573例常规CT检查之前被诊断为GC的患者。总共有30.66%(37,415例中的11,472例)来自门诊和急诊科和体格检查中心,而39.34%(37,415例中的25,943例)患者来自患处。
图像获取和处理
在胃镜检查之前,收集了训练队列中的CT图像,内部验证队列和外部验证队列。胃部分割是通过半监督的自我训练方法,结合了公共可用数据集获得的35带有手动注释的口罩和我们的内部训练集。使用ITK-SNAP软件(v.3.8,可在http://www.itksnap.org)36。由于可以轻松地将GC与门户静脉相CT图像中的正常胃组织区分开,因此我们划定了二维切片,其中含有肿瘤,以有效地概述静脉相相CT图像中的肿瘤边界。如果在两个放射科医生之间出现了ROI的划分中明显差异的情况下,通过建设性讨论实现了解决方案,从而达成共识。为了获得非对比度图像上的肿瘤注释,我们采用了契约37注册算法将静脉相形图像与非对比度图像对齐。然后将所得的变形场应用于静脉相的注释,从而为非对比相产生转化的肿瘤注释。Finally, the transferred tumor annotations on the noncontrast phase were again delineated and confirmed via the same procedure as on the venous phase.
AI model: GRAPE
GRAPE model was developed to analyze 3D noncontrast CT scans aimed at the detection and segmentation of GC.The model yields two outputs: a pixel-level segmentation mask outlining the stomach and potential tumors, and a classification score that categorizes patients into those with GC and normal controls.The model employs a two-stage strategy.The first stage involves the localization of the stomach region within the entire CT scan.This stage is implemented using a segmentation network, nnUNet38, with the configuration for 3D full resolution.The output stomach segmentation mask is used to generate a 3D bounding box of the stomach region, which is cropped and served as input for the second stage.This cropping enhances the efficiency and simplification of the subsequent stage by focusing the tumor segmentation specifically on the area of interest.In the second stage, a joint classification and segmentation network with dual branches is employed.The segmentation branch focuses on segmenting tumors in this identified stomach region.Meanwhile, the classification branch, which integrates multilevel feature maps from the segmentation branch, is designed to classify the patients as either having GC or as normal controls.Whereas noncontrast CT has inherent resolution limitations (~0.5–1 mm), GRAPE detects early GCs not through direct lesion visualization but by integrating subtle 3D morphometric patterns (for example, local wall thickening, mucosal heterogeneity) and contextual radiological features (for example, perigastric fat stranding, lymph node microcalcifications).
To address variations in CT imaging parameters across several centers, we adopted a robust preprocessing pipeline as follows.First, all scans were resampled to the median voxel spacing of the training dataset, specifically 0.717 mm × 0.717 mm × 5.0 mm, to harmonize differences in slice thickness and spatial resolution.Next, all intensity values were normalized using the mean and s.d.calculated from the training dataset to minimize interscanner intensity discrepancies.During training, fixed-size 3D patches with a size of 256 × 256 × 28 were extracted randomly from the resampled volumes, and random Gaussian noise was injected before feeding into the deep network to increase model robustness.At test time, predictions were generated using a sliding window strategy across the entire resampled volume, ensuring consistent performance regardless of original scan dimensions.
The training procedure involved a fivefold cross-validation approach by dividing the training set into five folds.Five models were trained individually using four folds, with validation on the one remaining fold, following the standard nnUNet cross-validation protocol.In the testing phase, ensemble learning was applied, averaging classification probabilities across five models to determine the classification score, and using pixel-level voting to determine segmentation output.The class with the higher probability between the two categories was chosen as the final classification for each test case.In addition, different status of stomach filling is challenging for the recognition of GC.GRAPE inherently addresses stomach filling variability through comprehensive training on a wide range of gastric volumes (fasting, <100 cm3to over-distended, >800 cm3)。
An advantage of the GRAPE model is its inherent interpretability.Given that it produces both segmentation and classification outputs, the segmentation output provides a pixel-level visual map that aids in understanding and confirming the classification results.To further enhance interpretability, we visualized the heatmap of the convolutional feature map in the classification branch using Grad-CAM (Gradient-weighted Class Activation Mapping)39, which is a widely applicable technique to identify the regions contribute most importantly to the classification.
评估指标
Our main goal is a binary classification task to determine whether a patient has GC.A GRAPE score greater than 0.5 is considered as the ‘high risk’ category for calculating AUC, sensitivity, specificity and accuracy.Furthermore, we access the proportion of GC detection stratified by T stage, TNM stage, lesion location and stomach filling during CT examination.
Reader studies
The aim of the reader study was to assess the difference in performance between GRAPE and radiologists in detecting GC on noncontrast CT.A total of 13 radiologists were enrolled in this study, comprising 5 senior radiologists and 8 junior radiologists.Details of the radiologists is shown in Extended Data Table3。The study comprised two sessions.In the first session, GRAPE’s performance was compared with that of radiologists with varying levels of expertise in GC imaging.The second session evaluated GRAPE’s potential to assist radiologists where we provided the radiologists with GRAPE’s prediction in addition to the noncontrast image.A washout period of at least 1 month was maintained between the 2 sessions for each radiologist.
统计分析
The performance of the GC and NGC classification was evaluated using the AUC, sensitivity, specificity, positive predictive value and balanced accuracy.Confidence intervals were calculated based on 1,000 bootstrap replications of the corresponding data.The significance comparisons of sensitivity, specificity and balanced accuracy were conducted using permutation tests to calculate two-sidedp values with 10,000 permutations.The threshold to determine statistical significance isp < 0.05.Data analysis was conducted in Python using the numpy (v.1.26.2), scipy (v.1.11.4) and scikit-learn (v.1.3.2) packages.
报告摘要
Further information on research design is available in theNature Portfolio Reporting Summarylinked to this article.
数据可用性
The datasets analyzed in this study are not publicly available due to restrictions imposed by the respective IRBs.Researchers may request access to the anonymized data and supporting clinical documentation by contacting corresponding author X.C.(chengxd@zjcc.org.cn).Access will be granted subject to IRB approval, a signed data-use agreement and will be for noncommercial academic purposes only.Requests will be processed within 6 weeks.
代码可用性
The code used for the implementation of GRAPE has dependencies on internal tooling and infrastructure and is under patent protection (CN116188392;the other application number is not currently in the public domain), and thus is not able to be publicly released.All experiments and implementation details are described in sufficient detail in方法to support replication with nonproprietary libraries.Several principal components of GRAPE are available in open-source repositories: PyTorch (https://pytorch.org/,v2.2.0) and nnU-NetV1 (https://github.com/MIC-DKFZ/nnUNet/tree/nnunetv1)。
参考
Smyth, E. C., Nilsson, M., Grabsch, H. I., van Grieken, N. C. & Lordick, F. Gastric cancer.柳叶刀 396, 635–648 (2020).
文章一个 CAS一个 PubMed一个 Google Scholar一个
Arnold, M. et al.Is gastric cancer becoming a rare disease?A global assessment of predicted incidence trends to 2035.肠 69, 823–829 (2020).
文章一个 PubMed一个 Google Scholar一个
Lin, J.-L.等。Global incidence and mortality trends of gastric cancer and predicted mortality of gastric cancer by 2035.BMC公共卫生 24, 1763 (2024).
文章一个 PubMed一个 PubMed Central一个 Google Scholar一个
Xia, J. Y. & Aadam, A. A. Advances in screening and detection of gastric cancer.J. Surg。Oncol。 125, 1104–1109 (2022).
文章一个 PubMed一个 PubMed Central一个 Google Scholar一个
Jiang, Y. et al.Predicting peritoneal recurrence and disease-free survival from CT images in gastric cancer with multitask deep learning: a retrospective study.Lancet Digit Health 4, e340–e350 (2022).
文章一个 CAS一个 PubMed一个 Google Scholar一个
Sun, D. et al.The effect of nationwide organized cancer screening programs on gastric cancer mortality: a synthetic control study.胃肠病学 166, 503–514 (2024).
文章一个 PubMed一个 Google Scholar一个
Allemani, C. et al.Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries.柳叶刀 391, 1023–1075 (2018).
文章一个 PubMed一个 PubMed Central一个 Google Scholar一个
Xia, R. et al.Estimated cost-effectiveness of endoscopic screening for upper gastrointestinal tract cancer in high-risk areas in China.JAMA Netw.打开 4, e2121403 (2021).
文章一个 PubMed一个 PubMed Central一个 Google Scholar一个
Hu, Y. et al.Performance evaluation of four prediction models for risk stratification in gastric cancer screening among a high-risk population in China.Gastric Cancer 24, 1194–1202 (2021).
文章一个 CAS一个 PubMed一个 Google Scholar一个
Wang, Z. et al.Nationwide gastric cancer prevention in China, 2021–2035: a decision analysis on effect, affordability and cost-effectiveness optimisation.肠 71, 2391–2400 (2022).
文章一个 PubMed一个 Google Scholar一个
Pickhardt, P. J. et al.Opportunistic screening: radiology scientific expert panel.放射学 307, e222044 (2023).
文章一个 PubMed一个 Google Scholar一个
Pickhardt, P. J. et al.Automated CT biomarkers for opportunistic prediction of future cardiovascular events and mortality in an asymptomatic screening population: a retrospective cohort study.Lancet Digit Health 2, e192–e200 (2020).
文章一个 PubMed一个 PubMed Central一个 Google Scholar一个
McWilliams, A. et al.Probability of cancer in pulmonary nodules detected on first screening CT.N. Engl。J. Med。 369, 910–919 (2013).
文章一个 CAS一个 PubMed一个 PubMed Central一个 Google Scholar一个
Prosper, A. E., Kammer, M. N., Maldonado, F., Aberle, D. R. & Hsu, W. Expanding role of advanced image analysis in CT-detected indeterminate pulmonary nodules and early lung cancer characterization.放射学 309, e222904 (2023).
文章一个 PubMed一个 Google Scholar一个
Cao, K. et al.Large-scale pancreatic cancer detection via non-contrast CT and deep learning.纳特。医学 29, 3033–3043 (2023).
文章一个 CAS一个 PubMed一个 PubMed Central一个 Google Scholar一个
Jiang, Y. et al.Radiographical assessment of tumour stroma and treatment outcomes using deep learning: a retrospective, multicohort study.Lancet Digit Health 3, e371–e382 (2021).
文章一个 CAS一个 PubMed一个 Google Scholar一个
Gillies, R. J. & Schabath, M. B. Radiomics improves cancer screening and early detection.癌症流行病。生物标志。上一条。 29, 2556–2567 (2020).
文章一个 CAS一个 Google Scholar一个
Wang, Z., Liu, Y. & Niu, X. Application of artificial intelligence for improving early detection and prediction of therapeutic outcomes for gastric cancer in the era of precision oncology.Semin Cancer Biol。 93, 83–96 (2023).
文章一个 CAS一个 PubMed一个 Google Scholar一个
Moor, M. et al.Foundation models for generalist medical artificial intelligence.自然 616, 259–265 (2023).
文章一个 CAS一个 PubMed一个 Google Scholar一个
Wang, Z., Mo, T.-M., Tian, L. & Chen, J.-Q.Gastrin-17 combined with CEA, CA12-5 and CA19-9 improves the sensitivity for the diagnosis of gastric cancer.Int J. Gen. Med 14, 8087–8095 (2021).
文章一个 CAS一个 PubMed一个 PubMed Central一个 Google Scholar一个
Sorscher, S.幽门螺杆菌幽门螺杆菌and gastric cancer screening.J. Clin。Oncol。 42, 3162–3163 (2024).
文章一个 PubMed一个 Google Scholar一个
Cai, Q. et al.Development and validation of a prediction rule for estimating gastric cancer risk in the Chinese high-risk population: a nationwide multicentre study.肠 68, 1576–1587 (2019).
文章一个 CAS一个 PubMed一个 Google Scholar一个
Xu, Z. et al.有效的血浆代谢指纹作为胃癌诊断和预后的新工具:一项大规模的多中心研究。肠 72, 2051–2067 (2023).
文章一个 CAS一个 PubMed一个 Google Scholar一个
Yu, P. et al.Genomic and immune microenvironment features influencing chemoimmunotherapy response in gastric cancer with peritoneal metastasis: a retrospective cohort study.Int J. Surg. 110, 3504–3517 (2024).
文章一个 PubMed一个 PubMed Central一个 Google Scholar一个
Roy, S. et al.Diagnostic efficacy of circular RNAs as noninvasive, liquid biopsy biomarkers for early detection of gastric cancer.摩尔。癌症 21, 42 (2022).
文章一个 CAS一个 PubMed一个 PubMed Central一个 Google Scholar一个
So, J. B. Y. et al.Development and validation of a serum microRNA biomarker panel for detecting gastric cancer in a high-risk population.肠 70, 829–837 (2021).
文章一个 CAS一个 PubMed一个 Google Scholar一个
Mok, J. W., Oh, Y. H., Magge, D. & Padmanabhan, S. Racial disparities of gastric cancer in the USA: an overview of epidemiology, global screening guidelines, and targeted screening in a heterogeneous population.Gastric Cancer 27, 426–438 (2024).
文章一个 PubMed一个 Google Scholar一个
Yu, P. et al.Multi-dimensional cell-free DNA-based liquid biopsy for sensitive early detection of gastric cancer.Genome Med 16, 79 (2024).
文章一个 CAS一个 PubMed一个 PubMed Central一个 Google Scholar一个
Zhu, X. et al.Development, validation, and evaluation of a risk assessment tool for personalized screening of gastric cancer in Chinese populations.BMC Med 21, 159 (2023).
文章一个 PubMed一个 PubMed Central一个 Google Scholar一个
Aberle, D. R. et al.Results of the two incidence screenings in the National Lung Screening Trial.N. Engl。J. Med。 369, 920–931 (2013).
文章一个 CAS一个 PubMed一个 PubMed Central一个 Google Scholar一个
Menon, U. et al.Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS).柳叶刀Oncol。 10, 327–340 (2009).
文章一个 PubMed一个 Google Scholar一个
Chan, K. C. A. et al.Analysis of plasma Epstein–Barr virus DNA to screen for nasopharyngeal cancer.N. Engl。J. Med。 377, 513–522 (2017).
文章一个 CAS一个 PubMed一个 Google Scholar一个
Chung, D. C. et al.A cell-free DNA blood-based test for colorectal cancer screening.N. Engl。J. Med。 390, 973–983 (2024).
文章一个 CAS一个 PubMed一个 Google Scholar一个
Zhang, M., Levine, S. & Finn, C. Memo: test time robustness via adaptation and augmentation.ADV。Neural Inf.过程。系统。 35, 38629–38642 (2022).
Gibson, E. et al.Automatic multi-organ segmentation on abdominal CT with dense V-networks.IEEE Trans。医学成像 37, 1822–1834 (2018).
文章一个 PubMed一个 PubMed Central一个 Google Scholar一个
Yushkevich, P. A., Yang, G. & Gerig, G. ITK-SNAP: an interactive tool for semi-automatic segmentation of multi-modality biomedical images.Annu.int。conf。IEEE Eng.医学生物。Soc。 2016, 3342–3345 (2016).
PubMed一个 Google Scholar一个
Heinrich, M. P., Jenkinson, M., Brady, M. & Schnabel, J. A. MRF-based deformable registration and ventilation estimation of lung CT.IEEE Trans。医学成像 32, 1239–1248 (2013).
文章一个 PubMed一个 Google Scholar一个
Isensee, F., Jaeger, P. F., Kohl, S. A. A., Petersen, J. & Maier-Hein, K. H. nnU-Net: a self-configuring method for deep learning-based biomedical image segmentation.纳特。方法 18, 203–211 (2021).
文章一个 CAS一个 PubMed一个 Google Scholar一个
Zhang, H. & Ogasawara, K. Grad-CAM-based explainable artificial intelligence related to medical text processing.生物工程 10, 1070 (2023).
文章一个 PubMed一个 PubMed Central一个 Google Scholar一个
致谢
We thank the International Scientific and Technological Cooperation Base for tumor molecular diagnosis and intelligent screening.X.C.was supported by The National Key Research and Development Program of China (2021YFA0910100) and Healthy Zhejiang One Million People Cohort (K-20230085).C.H.was supported by National Natural Science Foundation of China (82304946), postdoctoral Innovative Talent Support Program (BX2023375), China Postdoctoral Science Foundation (2023M733563) and Natural Science Foundation of Zhejiang Province (LMS25H160006).Z.X.was supported by National Natural Science Foundation of China (82473489), Medical Science and Technology Project of Zhejiang Province (WKJ-ZJ-2323) and Natural Science Foundation of Zhejiang Province (LBD24H290001);S.P. was supported by National Natural Science Foundation of China (82403546).
道德声明
竞争利益
Alibaba Group has filed for patent protection (CN116188392;the other application number is not currently in the public domain) for the work related to the methods of detection of GC in noncontrast CT.Y.X., Z.Z., Jianwei Xu, Z.Q., T.L., B.Y., J.Y., W.G., J.Z.and Ling Zhang are employees of Alibaba Group and own Alibaba stock as part of the standard compensation package.其他作者没有宣称没有竞争利益。
同行评审
Peer review information
自然医学thanks Florian Lordick and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.Primary Handling Editor: Lorenzo Righetto, in collaboration with the自然医学团队。
附加信息
Publisher’s noteSpringer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
扩展数据
Extended Data Fig. 1 The GRAPE model and its interpretability analysis.一个。
Model workflow and architecture.The GRAPE model takes the input of a non-contrast CT scan and first segment the stomach with a U-Net to obtain the ROI of the stomach region.It then processes the ROI region with a joint segmentation and classification network which extracts the multi-level feature of a U-Net backbone and perform classification after global pooling (GP) and fully connected layers (FC).b。Examples of interpretability analysis and three GC cases.The GRAPE model outputs the localization of the detected GC and aligns well with its heatmap visualization via the Grad-CAM approach.
Extended Data Fig. 2 ROC curves of individual centers.
The ROC curves show the performance of GRAPE in centers with more than 100 participants in the external validation cohort.
Extended Data Fig. 3 The performance of GRAPE in the internal and external cohorts.一个。
The sensitivity, specificity and AUC of GRAPE in centers with more than 100 participants in the external validation cohort.b -e。The sensitivity of GRAPE in GC detection stratified by T stages and tumor locations.f。The sensitivity of GRAPE in GC detection in different status of stomach filing.g。The sensitivity of GRAPE in GC detection stratified by TNM stage.h。The sensitivity of GRAPE in GC detection in patients younger or older than 60.我。The sensitivity of GRAPE in GC detection stratified by sex.Subgroups with less than 10 samples were omitted.
补充信息
权利和权限
开放访问This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material.您没有根据本许可证的许可来共享本文或部分内容的改编材料。The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material.If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.要查看此许可证的副本,请访问http://creativecommons.org/licenses/by-nc-nd/4.0/。Reprints and permissions
引用本文
Hu, C., Xia, Y., Zheng, Z.
等。AI-based large-scale screening of gastric cancer from noncontrast CT imaging.Nat Med(2025)。https://doi.org/10.1038/s41591-025-03785-6
已收到:
公认:
出版:
doi:https://doi.org/10.1038/s41591-025-03785-6