- Research
- Open access
- Published:
Orphanet Journal of Rare Diseases volume 20, Article number: 365 (2025) Cite this article
Abstract
Background
Rare bone diseases (RBDs) are an important group of conditions characterized by abnormalities in bone and cartilage. Their large number, individual rarity, and heterogeneity make accurate and timely diagnosis challenging. Establishing correlations between genotype and phenotype (mainly via imaging) is critical for diagnosing RBDs. Image recognition artificial intelligence (AI) has the potential to significantly improve the diagnostic process by assisting healthcare providers to identify and differentiate imaging patterns associated with various RBDs. This survey study sought to assess the interest of various healthcare providers worldwide in utilizing an AI-based assistant tool for the differential diagnosis of RBDs.
Method
Survey data were collected from March to September 2024. The survey was performed online and the link was disseminated via direct email, newsletters, and flyers at scientific talks and conferences.
Results
We received 103 completed surveys, representing respondents from 27 different countries covering most global regions, but mostly from Europe, the United States, and Canada. The majority of the participants are physicians (n = 92, 89%) and primarily work at academic medical centers (n = 84, 81%). While each participant could select multiple specialties, the most frequent clinician types were medical geneticists, pediatricians, and endocrinologists, accounting for 71 (69%) of the respondents. Ninety-four (91%) of the respondents find imaging to be very or extremely important, and the majority (n = 84, 81%) consider X-rays to be the most important imaging modality. Although around half of the participants (n = 45) have concerns about AI-related errors and consider the explainability of AI algorithms to be very (42/103) or extremely (9/103) important, 81% of the respondents report that they are somewhat (n = 39) or extremely (n = 45) likely to consider integrating image recognition AI into their current diagnostic workflow.
Conclusions
Most survey participants are open to integrating image recognition AI into their RBD diagnostic workflow. However, concerns about AI-related errors, privacy, and model interpretability highlight the importance of transparent collaboration between developers and healthcare professionals throughout the development process to ensure that such technologies are clinically trustworthy and practically adoptable.
Background
According to the most recent Nosology of Genetic Skeletal Disorders [1], there are over 700 known rare bone diseases (RBDs) that involve over 500 different genes. Though these diseases are individually rare, they collectively affect a large number of individuals. As with most rare diseases, diagnosing RBDs is inherently challenging, often requiring extensive time and multiple clinical visits, a process that can be frustrating for patients, and which on average takes ~ 5 years [2]. Effective treatment hinges on precisely identifying the type of RBD, emphasizing the importance of addressing the current diagnostic gap. Moreover, accurate diagnosis can have economic and societal impact, as available therapies are often highly specific and may involve highly variable access and costs [3].
The current diagnostic gap involves two main challenges: (1) due to the large number of RBDs, even expert clinicians may have encountered a limited number of affected individuals, and there are a limited number of experts. The first line of contact is usually general practitioners such as family doctors or general pediatricians, who are not trained in the diagnosis and management of RBDs [4]. This can significantly delay the identification and referral of these patients to experts (usually medical geneticists). (2) Although advances in DNA sequencing techniques have significantly improved the diagnosis of genetic conditions, being able to identify the cause of RBDs can be challenging due to incomplete understanding of genetic causes and other etiologic factors.
The past few years have involved an enormous expansion in the use of artificial intelligence (AI) methods in virtually all medical fields [5, 6]; this trend is anticipated to accelerate in the coming years. One relevant area is Next Generation Phenotyping (NGP), which in this context refers to the application of advanced computer vision techniques to medical imaging data of individuals with genetic conditions. There are already AI-based NGP tools that assist in the diagnosis of genetic diseases with characteristic patterns affecting the face (DeepGestalt [7] and GestaltMatcher [8]) as well as the eye (Eye2Gene [9]). These tools take images as input and provide a prioritized list of disorders (and/or disease-causing genes) that can help steer the differential diagnosis. At the heart of these technologies are advanced deep convolutional neural networks, which are trained using thousands of images from individuals with different rare disorders.
This same concept underlies the Bone2Gene AI, currently under development. Bone2Gene will be trained to identify and distinguish the unique imaging patterns linked to various RBDs. Initially, Bone2Gene AI will focus on training, using dorsopalmar radiographs of hands and wrists from various RBDs. This approach is selected because performing a hand X-ray for bone age assessment [10] is a common practice for children suspected of having bone irregularities. In subsequent stages, Bone2Gene will broaden its training to include radiographs from other areas of the body. As of this writing, the Bone2Gene dataset comprises approximately 2,700 hand X-rays from over 800 patients, covering more than 30 RBDs. The Bone2Gene team is currently developing multiple algorithms aiming at (i) detecting dysmorphic features in a hand X-ray, (ii) differentiating different patterns and outputting a list of most probable RBDs, and (iii) measuring different features of carpal, metacarpals, and phalangeal bones. The goal of all these algorithms is to provide the clinicians with relevant information for the differential diagnosis. See the Bone2Gene website (https://bone2gene.org) for current information.
While there is considerable current attention to AI, the attitudes and expectations of clinicians are critical to gauge prior to implementation. Following an approach inspired by the Lean Startup methodology [11, 12], which emphasizes the pivotal role of user feedback in shaping technological innovations, we aimed to gauge the interest of different healthcare providers across the world in having an AI-based assistant tool for the differential diagnosis of RBDs.
Method
The questionnaire
The survey included different groups of questions. First, we asked about participants’ demographics, specialties, the patient age groups they work with, and participant clinical experience with RBDs. Next, we collected data about the most frequent type of RBDs for which the participants provide care for. For that, we asked the participants to mark all the groups (according to the most recent revision of the nosology of RBDs [1]) that they or their healthcare facility have dealt with.
Further, we gauged the opinion of the participants on the importance of medical images (X-rays, MRI, etc.) and also the most important imaging modality for the postnatal diagnosis of RBDs. We also asked the participants how difficult they think it is to delineate between different RBDs based on visual inspection of patients’ radiographs (excluding the disorders with highly characteristic features such as achondroplasia).
In addition, we asked survey participants about their concerns regarding regulatory considerations, potential errors, and the importance of the explainability of AI. And finally, we gauged the perceived utility of AI-based diagnostic tools.
A list of the survey questions and the response options is provided in Table 1.
Participant recruitment and data collection
Survey data were collected from March to September 2024. To identify participants, we obtained names of clinicians associated with current RBD clinics (identified through our network and through web-based searches) as well as through relevant publications. Participants were recruited directly via email (n = 370) by providing a link to a survey from Qualtrics (Provo, Utah, United States) or indirectly via a flyer at scientific talks and conferences (such as the Annual Clinical Genetics Meeting, European Society of Human Genetics Conference, and International Skeletal Dysplasia Society Meeting). The survey link was also distributed via the newsletters of the European Reference Network on Rare Bone Diseases (ERN-BOND, with 50 HCPs as of this writing) and the European Reference Network for Rare Malformation Syndromes, Intellectual and Other Neurodevelopmental Disorders (ERN-ITHACA, with 71 HCPs as of this writing) in March and April 2024, respectively. The only inclusion criteria was involvement in rare bone disease patient care, which was asked by the first question and could also be assessed with follow-up questions. While an open link was provided, the research team verified no duplicate entries were included in the analysis. The survey response rate was calculated by the number of surveys completed divided by the number of participants directly contacted. We followed the Consensus-Based Checklist for Reporting of Survey Studies (CROSS) [13].
Results
Of the 125 participants who began the survey (provided demographic information), 103 completed the survey and provided information about their clinical experience with RBDs (27.8% response rate). The following results are based on these 103 responses. Table 1 provides the aggregated result to the survey questions and interactive visualizations of the results are also provided on the Bone2Gene website.
Demography
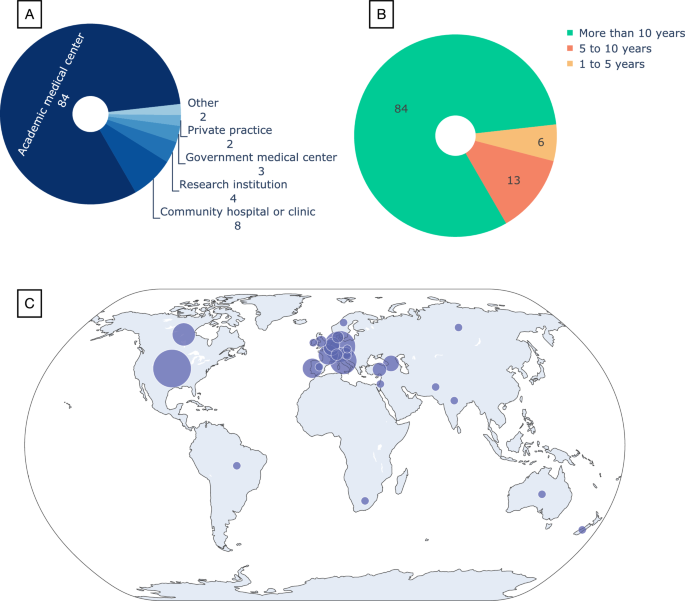
Out of a total of 103 complete survey responses 23 individuals (22%) describe themselves as involved in the diagnostic process, 6 (6%) in pre- and post-diagnostic care, and 69 (67%) are involved in both. The remaining 5 (5%) are only involved in research activities in this field. Ninety-two (89%) of the participants are physicians. The majority of participants (84, 81%) primarily work at academic medical centers (Fig. 1A) and have more than 10 years of experience (also 84, 81%, Fig. 1B). Ninety-six (93%) of the respondents are involved in teaching or training other healthcare professionals (Table 1 and Supplementary Fig. 1).
Demographics of the 103 participants who completed the survey. A Type of healthcare facility best describing where they primarily work. B Years of experience in the healthcare field. C The distribution of participants on the world map; the circle sizes reflect the number of participants (see the Supplementary information for the exact number per each of the 27 countries)
Survey participants represented most global regions (Fig. 1C) and a total of 27 different countries (Supplementary Fig. 2). Fifty-eight (56%) of the respondents are located in Europe and 30 (29%) are in the United States and Canada.
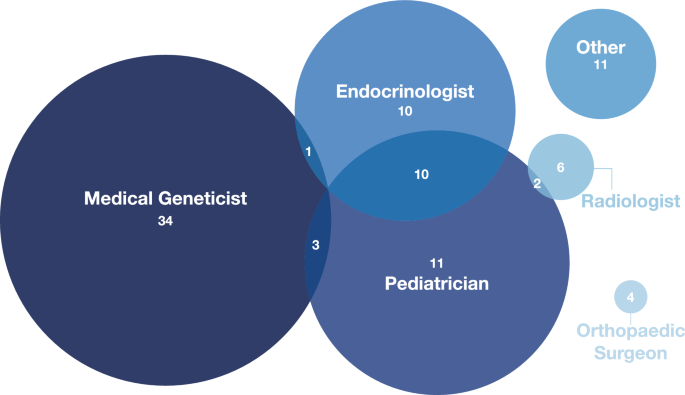
The diagram in Fig. 2 shows the distribution of the responses from the 92 physician participants to the question “Which title best describes your position?”. Participants could indicate multiple specialties. The most frequent groups were medical geneticists, pediatricians, and endocrinologists, together accounting for 71 (69%) of the respondents.
Specialties of the physician participants (n = 92). The most common specialty represented amongst participants was medical genetics. Some participants selected multiple specialties (e.g., endocrinologists who also practice pediatrics, n = 10). Other specialties represented include nephrologist, internist, rheumatologist, pediatric physiatrist, maternal–fetal medicine, general surgeon, pathologist, and geriatrician. We note that countries may have variable requirements regarding initial (general) training versus allowing clinicians to enter a subspecialty immediately, and some clinicians may have responded regarding their current practice rather than their entire training
Patients statistics
In this section, we report the responses to the questions related to the RBD patients seen by the participants. Fifty respondents (48%) see patients from all four age groups described in our questionnaire, namely 0–1,1–10,10–18, and greater than 18 years old. Further, most of the respondents see patients below 18 years old but 63 (61%) of them also see patients 18 years old or above (Table 1 and Supplementary Fig. 3).
The number of patients with known or suspected rare bone diseases seen by the facilities where the respondents work follows a roughly bell-shaped distribution (Supplementary Fig. 3). Sixty-four (62%) of the facilities see 10–100, seventeen (16%) see less than 10, and twenty-one (20%) see more than 100 patients per month.
According to the most recent revision of the nosology of RBDs [1] there are 41 different groups of skeletal disorders. In one of the questions in our survey, we asked the participants to mark all the groups that they or their healthcare facility have dealt with. The results are visualized in Supplementary Fig. 4. The most frequently observed disorder was the Osteogenesis imperfecta and bone fragility group, with 89% (n = 92) of participants reporting care of patients with this group of conditions at their institution. This was followed by Disorders of bone mineralization (n = 89, 86%) and FGFR3 chondrodysplasias (n = 88, 85%).
Role of imaging in diagnosing RBDs
Ninety-four (91%) of the respondents find imaging to be very or extremely important, and the majority (n = 84, 81%) consider X-rays to be the most important imaging modality (Table 1 and Supplementary Fig. 5).
Further, our result (Table 1 and Supplementary Fig. 6) shows that most participants, 83% (n = 86) regardless of years of experience, find the interpretation of patients’ radiographs and delineation of different RBDs based on visual inspection to be somewhat (n = 59) or extremely difficult (n = 27).
Participants’ perception of the use of AI
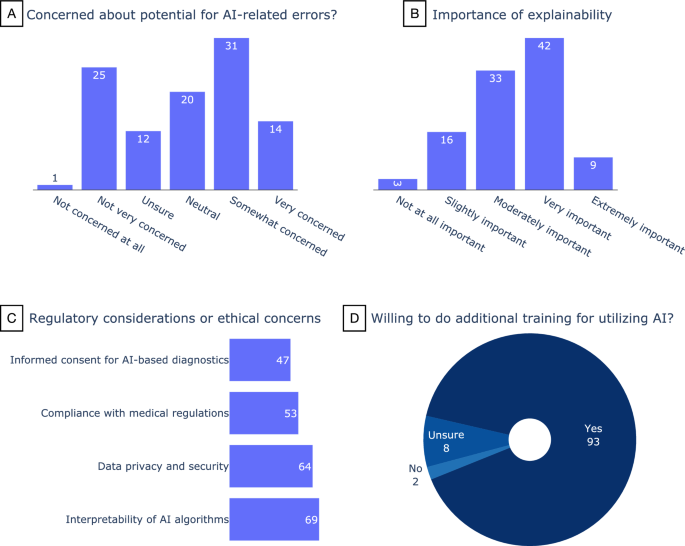
Forty-four percent of the participants (45/103) are somewhat (n = 31) or very (n = 14) concerned about AI-related errors, while 24% (n = 25) are not very concerned (Fig. 3A). Around half of the participants (n = 51) consider the explainability of AI algorithms to be very (42/103) or extremely (9/103) important (Fig. 3B). There was no relation between the years of experience of the participants and their answers to these questions.
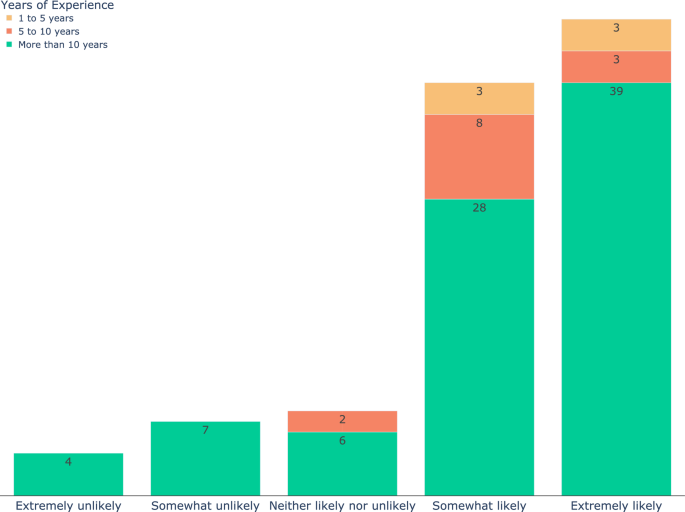
On the subject of regulatory considerations or ethical concerns (Fig. 3C), the interpretability of AI algorithms received the most attention (n = 69) followed by data privacy and security (n = 64). Ninety-three (90%) of the participants indicated they would be willing to do additional training for utilizing AI (Fig. 3D), and when asked how likely they are to consider integrating image recognition AI into their current diagnostic workflow, 81% responded they were somewhat (n = 39) or extremely (n = 45) likely (Fig. 4). Forty-five participants expressed interest in further collaborations in this area.
The responses of the participants to the question “If an image recognition AI is developed that provides you with a prioritized list of syndromes based on a radiograph, how likely are you to consider integrating it into your current diagnostic workflow?”. The color code shows the years of experience. Eighty-one percent of the participants (including all those with 1–5 years of experience) responded somewhat (n = 39) or extremely (n = 45) likely
Discussion
To address the need for a multidisciplinary approach [14, 15], it is crucial to gather insights from a wide range of specialties involved in the diagnosis and management of RBDs. We primarily targeted clinicians for participation, which would encompass most but not all persons involved in clinical care. Further, while most of the participants in our survey were medical geneticists, pediatricians, and endocrinologists, our survey also managed to reach radiologists, orthopedic surgeons, nephrologists, internists, rheumatologists, pediatric physiatrists, maternal–fetal medicine, general surgeons, pathologists, and geriatricians. However, a limitation of our study is the underrepresentation of the latter specialist groups, likely due to our recruitment strategy, which primarily targeted geneticists and endocrinologists. In particular, it is very important to gauge the opinion of radiologists on the use of AI tools for analyzing radiographs. Multiple studies in the literature have addressed this topic [16,17,18,19] reporting a generally positive attitude of radiologists towards AI. However, these studies have gauged the general expectations of radiologists and were not focused on RBDs. Future survey studies should try to reach more radiologists who are active in the field of RBDs.
International collaboration plays a crucial role in advancing research and development for rare diseases [20]. Our survey managed to gather data from participants from 27 countries around the world. The majority of the participants (n = 88, 85%) are located in Europe, United States, and Canada. However, despite trying to share the survey link widely via our network, we unfortunately received no responses from East and Southern Asia. Further, we received very few responses from West Asia (Middle East), Africa, Australia, and South America, hence, our sample is not representative of HCPs in those regions. This highlights the need for larger efforts to expand cross-regional connections and collaborations, including addressing issues such as language barriers (our survey was only offered in English) and different medical system practices [21].
Despite advances in new sequencing technologies and efforts at variant classification, many sequencing findings remain variants of uncertain significance (VUS), interpretations of which may require phenotypic examinations by trained dysmorphologists [22], and establishing correlations between genotype and phenotype (e.g., radiologic findings) can be essential for the final diagnosis [23]. Our results indicate that the majority of respondents find imaging (and in particular radiographs) to be highly important.
The opportunities and challenges of using AI in medicine and healthcare, in particular ethical concerns, privacy issues, and interpretability and explainability of AI models, have been discussed in various studies [22, 24,25,26]. Respondents to our survey echoed these concerns, although they generally expressed a positive attitude toward the use of AI.
Similar to the results from [27] reporting interviews with 20 different stakeholders about the adoption of facial recognition algorithms for rare disease diagnosis, the majority of the participants of our survey are also very likely to consider integrating an image recognition AI into their current RBD diagnostic workflow and to undergo further training for using AI.
Conclusion
In this study, we conducted a survey to gather feedback from potential users of an AI-based diagnostic tool. The aim was to integrate their input into the development process and reduce the information asymmetry between AI researchers and clinicians, who possess essential insights into the practical challenges and needs of diagnosing RBDs. Most survey respondents indicated they would be willing to incorporate AI into their diagnostic workflow for RBDs. However, AI-related errors, privacy issues, and interpretability and explainability of AI models remain the most important concerns of healthcare professionals regarding AI adoption. This highlights the need for close and transparent collaboration between developers and healthcare professionals before and during the design, testing, and implementation of AI-based tools.
Data availability
The datasets generated during the current study are not publicly available due preserving the privacy of individual survey participants, but the aggregated results used for analysis and producing the figures are provided in Table 1 of the manuscript and are also provided as a supplementary Excel sheet.
References
Unger S, Ferreira CR, Mortier GR, Ali H, Bertola DR, Calder A, et al. Nosology of genetic skeletal disorders: 2023 revision. Am J Med Genet A. 2023;191:1164–209. https://doi.org/10.1002/ajmg.a.63132.
Molster C, Urwin D, Di Pietro L, Fookes M, Petrie D, van der Laan S, et al. Survey of healthcare experiences of Australian adults living with rare diseases. Orphanet J Rare Dis. 2016;11:30. https://doi.org/10.1186/s13023-016-0409-z.
Sabir AH, Cole T. The evolving therapeutic landscape of genetic skeletal disorders. Orphanet J Rare Dis. 2019;14:300. https://doi.org/10.1186/s13023-019-1222-2.
Groft SC, Posada M, Taruscio D. Progress, challenges and global approaches to rare diseases. Acta Paediatr. 2021;110:2711–6. https://doi.org/10.1111/apa.15974.
Rajpurkar P, Chen E, Banerjee O, Topol EJ. AI in health and medicine. Nat Med. 2022;28:31–8. https://doi.org/10.1038/s41591-021-01614-0.
Solomon BD, Cheatham M, de Guimarães TAC, Duong D, Haendel MA, Hsieh TC, et al. Perspectives on the current and future state of artificial intelligence in medical genetics. Am J Med Genet A. 2025. https://doi.org/10.1002/ajmg.a.64118.
Gurovich Y, Hanani Y, Bar O, Nadav G, Fleischer N, Gelbman D, et al. Identifying facial phenotypes of genetic disorders using deep learning. Nat Med. 2019;25:60–4. https://doi.org/10.1038/s41591-018-0279-0.
Hsieh T-C, Bar-Haim A, Moosa S, Ehmke N, Gripp KW, Pantel JT, et al. GestaltMatcher facilitates rare disease matching using facial phenotype descriptors. Nat Genet. 2022;54:349–57. https://doi.org/10.1038/s41588-021-01010-x.
Pontikos N, Woof W, Veturi A, Javanmardi B, Ibarra-Arellano M, Hustinx A, et al. Eye2Gene: prediction of causal inherited retinal disease gene from multimodal imaging using deep-learning. 2022 [cited 30 Mar 2023]. https://doi.org/10.21203/rs.3.rs-2110140/v1.
Rassmann S, Keller A, Skaf K, Hustinx A, Gausche R, Ibarra-Arrelano MA, et al. Deeplasia: deep learning for bone age assessment validated on skeletal dysplasias. Pediatr Radiol. 2024;54:82–95. https://doi.org/10.1007/s00247-023-05789-1.
Blank S, Eckhardt JT. The lean startup as an actionable theory of entrepreneurship. J Manage. 2024;50:3012–34. https://doi.org/10.1177/01492063231168095.
Felin T, Gambardella A, Novelli E, Zenger T. A scientific method for startups. J Manage. 2024;50(8):3080–104. https://doi.org/10.1177/01492063231226136.
Sharma A, Minh Duc NT, Luu Lam Thang T, Nam NH, Ng SJ, Abbas KS, et al. A consensus-based checklist for reporting of survey studies (CROSS). J Gen Intern Med. 2021;36:3179–87. https://doi.org/10.1007/s11606-021-06737-1.
Walleczek N-K, Förster K, Seyr M, Kadrnoska N, Kolar J, Wasinger-Brandweiner V, et al. Rare skeletal disorders: a multidisciplinary postnatal approach to diagnosis and management. Wien Med Wochenschr. 2021;171:94–101. https://doi.org/10.1007/s10354-021-00820-2.
Chandran M, Alves I, Carpenter T, Davis M, Hsiao EC, Petryk A, et al. Improving care pathways for people living with rare bone diseases (RBDs): outcomes from the first RBD summit. Osteoporos Int. 2023;34:1301–10. https://doi.org/10.1007/s00198-023-06791-x.
Coppola F, Faggioni L, Regge D, Giovagnoni A, Golfieri R, Bibbolino C, et al. Artificial intelligence: radiologists’ expectations and opinions gleaned from a nationwide online survey. Radiol Med. 2021;126:63–71. https://doi.org/10.1007/s11547-020-01205-y.
Adelsmayr G, Janisch M, Pohl M, Fuchsjäger M, Schöllnast H. Facing the AI challenge in radiology: lessons learned from a regional survey among Austrian radiologists in academic and non-academic settings on perceptions and expectations towards artificial intelligence. Digit Health. 2024;10:20552076241298470. https://doi.org/10.1177/20552076241298472.
Huisman M, Ranschaert E, Parker W, Mastrodicasa D, Koci M, de PintoSantos D, et al. An international survey on AI in radiology in 1041 radiologists and radiology residents part 1: fear of replacement, knowledge and attitude. Eur Radiol. 2021;31:7058–66. https://doi.org/10.1007/s00330-021-07781-5.
Zanardo M, Visser JJ, Colarieti A, Cuocolo R, Klontzas ME, Dos Pinto Santos D, et al. Impact of AI on radiology: a EuroAIM/EuSoMII 2024 survey among members of the European society of radiology. Insights Imaging. 2024;15:240. https://doi.org/10.1186/s13244-024-01801-w.
Julkowska D, Austin CP, Cutillo CM, Gancberg D, Hager C, Halftermeyer J, et al. The importance of international collaboration for rare diseases research: a European perspective. Gene Ther. 2017;24:562–71. https://doi.org/10.1038/gt.2017.29.
Sheng B. Connecting each other in rare diseases: A call for cross-regional collaboration. In: Fong BYF, Wong WCW, editors. Gaps and actions in health improvement from Hong Kong and beyond. Singapore: Springer; 2023. p. 391–400. https://doi.org/10.1007/978-981-99-4491-0_26.
Solomon BD, Adam MP, Fong C-T, Girisha KM, Hall JG, Hurst ACE, et al. Perspectives on the future of dysmorphology. Am J Med Genet A. 2022. https://doi.org/10.1002/ajmg.a.63060.
Handa A, Grigelioniene G, Nishimura G. Skeletal dysplasia families: a stepwise approach to diagnosis. Radiographics. 2023;43: e220067. https://doi.org/10.1148/rg.220067.
Decherchi S, Pedrini E, Mordenti M, Cavalli A, Sangiorgi L. Opportunities and challenges for machine learning in rare diseases. Front Med. 2021;8: 747612. https://doi.org/10.3389/fmed.2021.747612.
Adlung L, Cohen Y, Mor U, Elinav E. Machine learning in clinical decision making. Med. 2021;2:642–65. https://doi.org/10.1016/j.medj.2021.04.006.
Amann J, Blasimme A, Vayena E, Frey D, Madai VI. Explainability for artificial intelligence in healthcare: a multidisciplinary perspective. BMC Med Inform Decis Mak. 2020;20:1–9. https://doi.org/10.1186/s12911-020-01332-6.
Hallowell N, Badger S, McKay F, Kerasidou A, Nellåker C. Democratising or disrupting diagnosis? Ethical issues raised by the use of AI tools for rare disease diagnosis. SSM–Qual Res Health. 2023;3: 100240. https://doi.org/10.1016/j.ssmqr.2023.100240.
Acknowledgements
We would like to thank all the participants for completing our survey and ERN-BOND and ERN-ITHACA for distributing the survey via their newsletters.
Funding
Open Access funding enabled and organized by Projekt DEAL. The Bone2Gene project is funded by the Go-Bio initial program from the German Federal Ministry of Education and Research (BMBF). This work was supported in part (salary support for RLW and BDS) by the Intramural Research Program of the National Human Genome Research Institute and the National Institutes of Health.
Ethics declarations
Ethics approval and consent to participate
The study was approved as IRB-exempt by the National Institutes of Health IRB (IRB# 002077).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Javanmardi, B., Waikel, R.L., Tkemaladze, T. et al. Artificial intelligence for diagnosing rare bone diseases: a global survey of healthcare professionals. Orphanet J Rare Dis 20, 365 (2025). https://doi.org/10.1186/s13023-025-03875-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13023-025-03875-1