抽象的
骨科中常规的诊断和治疗方法通常是时间密集的,并且与诊断错误率升高有关,从而迫切需要更有效的工具来改善当前状况。最近,人工智能(AI)越来越多地整合到骨科实践中,提供了支持数据驱动的方法来支持诊断和治疗过程。随着AI技术及其纳入常规骨科工作流程的持续发展,对AI原则及其临床应用的全面了解变得越来越重要。审查始于AI的核心概念和历史演变的摘要,然后检查机器学习和为骨科临床和研究应用设计的深度学习框架。然后,我们探索骨科中的各种基于AI的应用,包括图像分析,疾病诊断和治疗方法,例如手术辅助,药物开发,康复支持和个性化治疗。这些应用旨在帮助研究人员和临床医生对AI在骨科中的当前应用有更深入的了解。该评论还强调了影响AI实际使用的主要挑战和局限性,例如数据质量,模型可推广性和临床验证。最后,我们讨论了改进AI技术并将其安全有效整合到骨科护理中的未来方向。
背景
近年来,人工智能(AI)经历了迅速的进展,通过模拟人类推理和帮助临床判断的能力来促进多个医学实践领域的变化[1]。2016年,据报道,在美国,可预防的医疗错误每年造成25万多人死亡[2]。在卫生保健中,AI持有巨大的希望,以提高护理质量并最大程度地减少此类错误[3,,,,4]。计算能力和数据访问的改善导致了AI技术和相关医疗设备的开发迅速增加,其中许多已获得美国食品和药物管理局的批准[5]。这些创新逐渐被整合到各种医学专业中。特别是,在骨科中,一个因采用技术进步而备受认可的领域,AI已开始应用于提高诊断准确性并改善患者的预后。
尽管AI越来越多地用于支持骨科中的早期诊断和精确治疗[6],将人工智能整合到骨科实践中提出了独特的挑战。骨科条件通常涉及复杂的生物力学系统,患者特异性的解剖变异以及长期恢复期,需要强大和精确的AI溶液的复杂性。诸如数据稀缺,非结构化临床信息的复杂性以及与当前工作流程的整合问题等挑战继续限制AI在医学中的实际使用。此外,确保患者数据的道德使用,法规依从性以及医疗保健提供者接受的接受进一步增加了复杂性。
尽管仍然存在挑战,但骨科中AI应用的各个方面仍表现出巨大的潜力。AI技术通过高级图像识别来实现更准确的诊断,通过预测分析优化手术干预措施,并使用特定于患者的数据来个性化治疗计划。特别是在手术应用中,基于AI的机器人技术和导航系统正在计划和执行骨科手术的方式,从而提高了精度和减少恢复时间[7,,,,8]。总的来说,这些创新与精确医学的更广泛目标相吻合[9],提供量身定制的干预措施,以满足个人患者需求。
近年来,对在骨科手术中应用AI的兴趣有所增加[6,,,,10,,,,11,,,,12]。这篇综述研究了AI在骨科诊断和治疗中的当前应用,重点是其临床益处,技术局限性以及需要进一步研究和开发的领域(R&D)。通过确定当前研究和实践中的差距,我们重点介绍了AI有可能增强骨科护理并指导未来为更有效整合AI技术的关键领域。
AI的定义
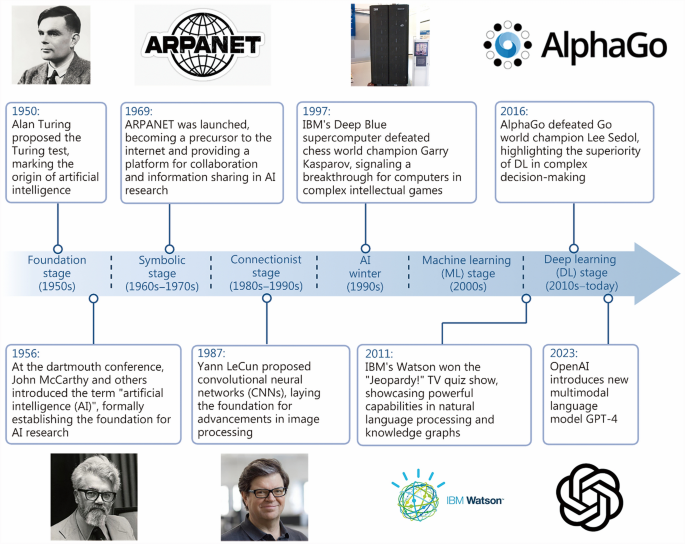
AI的发展一直是一个逐步的过程[13](图 1)。AI的基础可以追溯到1956年麦卡锡的作品,这标志着建造可以模仿人类思维的机器的开始的开始14,,,,15,,,,16,,,,17](图 2)。但是,直到1980年代,计算机化和流程的自动化使AI能够更广泛地发展[7]。自2000年代初以来,AI迅速发展。通过利用大型数据集,深度学习(DL)使机器能够执行需要更高级别的模式识别和抽象的高级任务[18],在自然语言处理,图像识别和语音识别方面取得了显着的结果。在过去的二十年中19,,,,20,,,,21]。在骨科中,AI的应用提高了诊断准确性,实现了个性化的治疗计划,并支持预测性建模,共同改善了患者的结果和经验。
机器学习(ML)
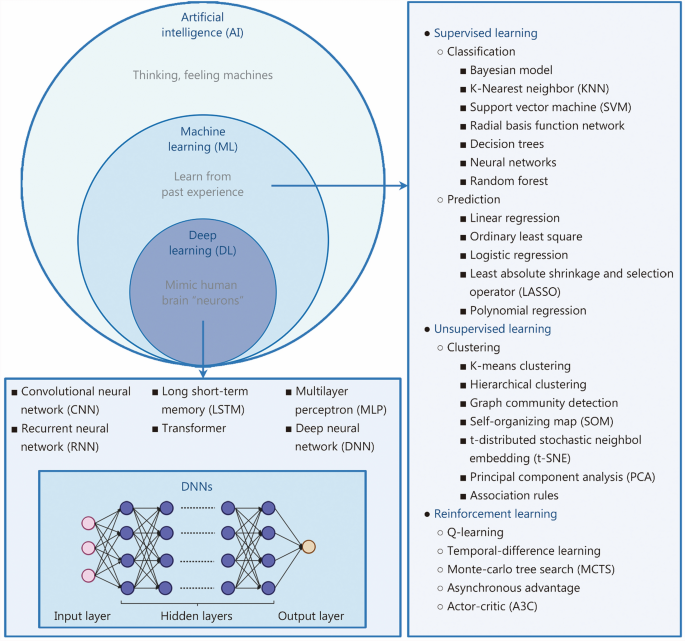
ML是AI的关键子集,它使计算机系统能够从经验中学习并从数据集中获取知识(图。 2)。ML利用算法通过识别输入数据中的模式来预测结果,并将这些预测与已知结果进行比较,以量化和完善算法的准确性[22]。ML以两种主要形式表现出来:监督和无监督的学习[22,,,,23,,,,24]。在监督学习中,通过标记的数据集对算法进行培训[25]。该模型学会根据提供的示例进行预测,从而实现自主决策。监督学习的重点是分类和预测[26]。相反,无监督的学习涉及在未标记的数据上培训算法,使它们能够独立发现隐藏的模式或结构[27]。无监督的ML技术通常用于聚类相似的数据点或识别数据集中变量之间的关系。
在医疗保健中,已广泛应用受监督和无监督的学习策略[26,,,,28]。监督的ML促进了预测模型的发展,增强了疾病检测和治疗结果预测等领域的能力[28,,,,29]。相反,无监督的ML技术可以在医疗保健数据中发现潜在模式,从而导致疾病亚型和患者分层等见解[26,,,,28]。最近的研究有效地采用了各种应用的监督学习方法,包括专业运动员之间的伤害风险预测,通过运动学分析评估膝关节骨关节炎(OA)[30,,,,31],以及临床相关结果的预测[32,,,,33,,,,34,,,,35,,,,36]。相反,无监督的学习已被用来识别不同的患者亚组,有助于风险分层和个性化治疗。最近的一项研究将无监督的聚类应用于膝盖韧带注册表数据,并确定了5例具有不同前交叉韧带(ACL)修订率的患者亚组。该分类基于年龄,移植类型和术前功能得分[37]。
DL
DL是一种多层表示学习方法,它通过模仿人脑神经元的结构将原始数据转化为抽象特征,从而实现自动模式识别和分类(图。 2)。DL利用人工神经网络从大型高维数据中提取复杂的特征[18]。这些网络由数百万分层排列的互连神经元组成。每个神经元从上一层接收输入,并将所处理的信息传递给下一个。在培训期间,在产生输出层的预测之前,将具有已知结果的数据馈入输入层并通过隐藏层处理[38]。
有几种基础类型的神经网络,每种都适合不同的数据类型和任务。多层感知构成了许多DL模型通过其完全连接的结构的基础[20]。复发性神经网络旨在通过一次处理一个元素来处理顺序数据,同时通过隐藏的状态保留先前输入的信息[39]。相反,卷积神经网络(CNN)对于图像分析特别有效,因为它们能够捕获局部空间特征[38]。这些模型通常是独立使用的,或在更高级的DL体系结构中用作组件。这些神经网络体系结构已在包括骨科在内的各个领域都取得了重大进步,在这些领域,DL已广泛应用于图像分析等任务[40,,,,41], 诊断 [42,,,,43,,,,44], 外科手术 [45],药物开发[46,,,,47,,,,48]和预测分析[49]。
用于骨科图像分析的算法开发
AI的最新发展促进了骨科成像方面的进展,尤其是在解剖结构的自动分割和定位方面(表) 1)[40,,,,41,,,,50,,,,51,,,,52,,,,53,,,,54,,,,55,,,,56,,,,57,,,,58]。AI提供了简化这些复杂任务的实用工具,并支持ML和DL技术在骨科条件的诊断和治疗中的应用。
分割和分类算法的进度
骨科图像分析受益于分割算法的完善,这些算法能够描绘出软骨,骨骼,肌肉和神经元素等结构。这些进步基于传统的ML模型和现代DL架构。
对于关节软骨分割,Shah等人。[58]利用经过验证的ML模型来量化健康膝盖的3910磁共振成像(MRI)数据集的软骨厚度。这种方法实现了组织层之间的区分,支持关节变性的纵向观察。但是,基于ML的模型通常缺乏适应性,并且它们对手动特征提取的依赖性限制了可扩展性。相反,Norman等。[41]实施了基于U-NET的DL模型,用于分割软骨和半月板。该模型的软骨达到了骰子系数在0.770至0.878之间,半月板的骰子系数为0.753和0.809,每扫描的分割时间平均为5。它还显示了与手动松弛和形态测量的密切相关性,这表明了其在临床工作流程中可靠评估的潜力(表格 1)。
对于骨结构分割,Ghidotti等人。[53]比较了U-NET和丝网膜网状结构,并达到了高空间精度。Segresnet具有增强的跳过连接,在特定的训练条件下表现出了出色的适应性,强调了其在骨科应用中的精确三维(3D)解剖模型的潜力。同样,在骨盆分割中,Hemke等人。[40]开发了一个深层卷积神经网络(DCNN)模型,能够将骨盆计算机断层扫描(CT)图像分割成多个组织类别,并以高精度为单位,从而在图形处理单元上完成了0.1以下的每个图像的分割。这些结果突出了该模型的鲁棒性和效率,增强了DL在综合人体组成分析中的价值(表 1)。脊柱细分,特别是在病理和不完整条件下,仍然具有挑战性。
早期方法,例如oktay和Akgul的马尔可夫链样模型,具有手工制作的定向梯度(PHOG)和图像投影描述符特征的手工锥体直方图,在异常磁共振数据集中的圆盘和椎骨定位[57]。在此基础上,Glocker等人。[54]和Burstrã¶m等。[50]引入了CT和锥形梁计算机断层扫描(CBCT)的自动化方法,以促进椎弓根螺钉导航。为了克服手工制作的功能的局限性,Jakubicek等人。[59]提出了一个创新的多阶段DL框架,该框架将3个CNN与新型的空间滤波和全局优化方法集成在一起,从而达到了4.4毫米和87.1%椎骨标记的平均椎间盘定位误差,这些误差在挑战性的病理学和不完整的3DDDCT脊柱扫描方面达到了精度。Fan等人补充了这些努力。[52]证明了对腰椎神经和骨骼的高临界性分割,其性能在统计学上与手动分割相当,从而增强了基于DL的模型的临床潜力。在整个研究中,多阶CNN框架比处理异常脊柱形态的其他框架具有更好的弹性(表 1)。
除了解剖学细分和本地化之外,AI还越来越多地应用于自动检测和分类任务,从而扩展了其在临床决策中的效用。Jamaludin等。[55]开发了一种基于CNN的系统,该系统在MR图像中获得了95.6%的盘式检测和标记,其病理分级预测非常匹配放射学家的评估,从而提供一致的体素级别证据验证热点,以支持临床解释。同样,Lind等人。[56]创建了一个基于RENET的神经网络,该网络对膝盖周围的49个嵌套裂缝类别进行了分类,在近端胫骨骨折的曲线下达到了加权平均面积(AUC)为0.87,pat骨骨折为0.89,远端FEMUR骨折为0.89,近75%的AUC估计值超过0.8。这些结果证明了基于射线照相数据的详细和可靠断裂分类的模型能力。Chae等人扩展为多模式数据。[51]组合成像和数字脚压力数据以对脚类型进行分类,证明了AI超出常规成像任务的适应性(表格 1)。
这些研究说明了AI在骨科诊断中的潜力和复杂性的增长,尤其是在整合了各种数据类型并解决了非典型病理时。尽管取得了这些进步,但仍有一些局限性和挑战仍有待解决,以确保各种临床方案的一致和准确的表现。
图像分析中的技术限制
尽管图像分割的最新进展及其在识别解剖结构和协助临床决策方面的准确性和效率提高,但在当前的研究中,仍有几种持续的局限性。这些局限性与数据依赖性,可推广性,模型设计和临床适用性有关。首先,许多调查面临着与注释准确性有关的困难[41,,,,56]。某些作品将手动细分作为参考标准,它本质上是主观的,并且在注释者之间缺乏一致性,从而将其有效性限制为地面真理[41]。仅依赖注释的放射学报告,而没有证实CT,MRI或手术发现的证据,这会增加错误分类的风险,尤其是在处理复杂的解剖结构或细粒度的亚分类[56]。此外,培训数据集的代表性和病理多样性通常不足。基于单个机构数据的研究可能会受到特定成像协议,患者人群和设备设置的影响,从而限制了其更广泛的适用性[40,,,,56]。此外,某些作品将分割的范围限制为单个解剖平面或特定结构,例如仅使用标准轴向髋关节切片评估肌肉质量[40]或仅分割L5/S1脊柱水平,该水平阻止了多视图或3D信息的整合以进行综合分析[41,,,,55]。例如,Bursträm等。[50]在没有明显病理学的尸体图像上训练了模型,尽管该模型对于一般的解剖学分割有效,但在脊柱畸形或退化性病变等疾病的情况下可能会表现不佳。
同样重要的是,评估指标缺乏标准化,这限制了整个研究的可比性。一些研究优先考虑分类的准确性,但忽略了一致性指标,例如KAPPA系数,可能掩盖了类别之间的性能差异,并限制了有关临床可靠性的透明度[55]。此外,仅对许多现有模型的临床实用性进行了初步评估,并且仍然需要通过未来的大规模,预期验证进行确认。例如,Lind等人。[56]提出的用于计划硬膜外类固醇注射的DL辅助平面定位,但其可行性仍有待通过广泛的试验确定。同样,Hemke等。[40]承认有必要验证癌症或慢性疾病患者肌肉质量评估模型的预后效用。
AI驱动的骨科诊断创新
诊断错误在手术护理和专业的所有阶段都发生,作为主要贡献者的临床决策和沟通崩溃,导致超过一半的病例至少造成适度的伤害,其中1例中有1例中有1例[49,,,,60]。AI技术的加速进步,再加上大量的医学数据[61],已将AI整合到诊断研究和临床应用中的增加[62]。
促进精确有效的断裂诊断
断裂诊断仍然是骨科实践的基石,但是由于观察者间的变异性,复杂的解剖结构和繁重的临床工作量,传统的放射线解释易于误诊。63,,,,64]。2019年,全球年龄标准化的骨折发病率估计为2296.2例每10万人[65],诊断错误会导致治疗延迟和功能恢复不良[44,,,,66]。将AI纳入断裂诊断是一个有希望的进步,旨在提高诊断精度,促进解释的一致性和简化临床过程[67,,,,68]。许多研究表明,基于AI的模型在检测急性裂缝方面表现出色,达到了与专家放射科医生相当的准确性(表格) 2)[42,,,,43,,,,44,,,,69,,,,70,,,,71,,,,72,,,,73,,,,74,,,,75,,,,76,,,,77,,,,78]。
在X射线和CT数据集中训练的AI模型已成功识别出不同尺寸和复杂性的断裂。在Chung等人的研究中。[42],DCNN表现出在检测肱骨近端骨折的熟练程度。同样,Lindsey等人。[44]训练有训练的深神经网络(DNN),能够在射线照片中检测和定位腕部骨折,其诊断精度与高级专业的整形外科医生相当。这项研究还表明,受过训练的模型的急诊医学临床医生的敏感性为92.5%,特异性为94.1%。Kim等人的另一项研究。[43]证明,转移学习是在医学数据集上对非医学图像进行微调的CNN进行了微调,即使标记有限的X光片也可以产生高度准确的断裂诊断。此外,Guan等人。[71]提出了一种用于基于X射线骨折的检测的新型DL方法,即使对于肌肉骨骼X光片(MURA)数据集,臂骨折检测的最新平均准确性也为62.04%。与研究基于高分辨率数据或更截然不同的解剖目标的研究相反,Guan等人的研究。[71]专注于克服现实世界成像的约束。在将AI模型应用于上肢断裂检测中后,AI模型还用于检测肋骨骨折,这是一项特别具有挑战性的任务,这是由于传统胸部X光片的高度高达50%,该任务受到传统的胸部射线照相的限制,该率受到重叠结构,读取器的重叠,读取者的变化和图像质量[图像质量[79,,,,80]。Niiya等。[75]引入了一个达到93.5%灵敏度的系统,从而显着提高了创伤环境中的断裂检测效率。同样,Yao等人。[78]使用三步算法构建了基于DL的肋骨断裂检测系统。
此外,AI在诊断胸骨椎骨骨折的诊断方面表现出了希望,由于胸甲骨折的肌肉骨折经常被遗漏或误诊,因为它们的次射线片次射线照相术,可能会导致治疗延迟,并且预后较差[81,,,,82]。此外,椎骨压缩骨折,尤其是涉及显着前高度恢复和低骨矿物质密度的骨折,不仅增加了随后邻近椎骨骨折的风险,而且还大大增加了未来髋部骨折的风险[83,,,,84]。因此,胸骨压缩骨折的早期诊断至关重要。Burns等人。[70]利用自动化的ML计算机系统来检测敏感性95.7%的脊柱骨折。另外,Li等人。[74]开发了一种基于AI的腰椎骨折检测系统,该系统表现出92%的精度,91%的敏感性和94%的特异性。
DL算法已广泛应用于多站点断裂检测。研究表明,CNN和Resnet模型在诊断股骨颈,臀部,scaphoid和Calcaneus的裂缝方面具有很高的精度[63,,,,73,,,,76,,,,77]。琼斯等。[72]报告针对16个解剖骨折部位的DL模型的AUC为0.974。灵敏度和特异性分别为95.2%和81.3%。在对具有完全专家一致的X光片的次要分析中,AUC进一步增加到0.993,该分析近似于经验丰富的放射科医生和骨科医生的诊断准确性。
尽管如此,基于AI的骨折诊断仍然面临几个尚未解决的挑战[85]。首先,现实世界中的多中心验证对于确保AI系统在临床环境中的性能是至关重要的,因为意外行为在标准评估过程中可能不会浮出水面[86]。其次,成本关注和缺乏透明度仍然是放射学采用AI的关键障碍。现实世界的临床效率和诊断质量的提高对于证明投资和促进更广泛的实施至关重要[87]。此外,由于训练暴露有限,当前的AI系统难以检测出细微或非典型的骨折,例如应激骨折或病理骨骼的骨折。此外,大多数AI工具都是针对特定任务(例如图像分类)而设计的,并且没有复制放射科医生的全部范围,其中包括临床相关性和复杂的决策[85]。
协助早期诊断出髋关节发育不良(DDH)
DDH是最常见的小儿肌肉骨骼疾病,如果未诊断和治疗,可能会导致严重的残疾。因此,及时,准确的检测对于保留髋关节功能和防止长期并发症至关重要[88,,,,89]。传统的诊断方法,例如针对婴儿的髋关节超声和年龄较大儿童的骨盆X光片,很大程度上依赖临床医生的专业知识,使它们既耗时,劳动力且易于变异[90]。AI在基于成像的DDH筛选中的应用有助于提高诊断准确性,并提高工作流程效率。Huang等。[91]引入了DDHNET,这是一种完全自动化的AI系统,可通过超声图像测量4个关键的髋部参数(± - 角,α-and,α-angle,股骨头覆盖范围和Pubofofoboral距离)。DDHNET的诊断精度为98.64%,特异性为100.00%,灵敏度为90.56%。这些结果证明了系统提供精确和一致的测量的能力,同时大大减少了分析所需的时间。Xu等。[92]进一步开发了一个AI模型,该模型衡量了其他参数,例如髋臼指数,中心角,Tã¶nnis等级和国际髋关节增生研究所(IHDI)等级,证明诊断性能与矫形器专家的诊断性能可比性相当,但与150. 30. 30. 3的外观相比,该模型仅降低了。经验水平(p<<0.001)。
AI还具有增加在不同临床环境中DDH筛查的访问权限的潜力。Jaremko等。[93]发表了一项研究,该研究表明,基于AI的超声评估软件程序Medo-Ihip(Medo.ai,加拿大埃德蒙顿,加拿大,2021年),使初级保健工作者可以接受简单的培训来筛选DDH的婴儿。这项研究表明,基于AI的便携式超声筛选可以实现类似于专业超声筛选的随访和病例检测率。MEDO-HIP促进了人口级的DDH筛查,这有望降低筛查成本并实现广泛的实施。
AI在客观测量中表现良好,但在主观评估中挣扎[92]。需要更多的培训数据来提高其在复杂情况下的能力。图像质量仍然是一个关键因素,因为质量较低的扫描可能导致结果不准确。AI系统高度取决于所有关键的解剖标志的存在。此外,大多数研究都是小规模的,并且依赖于特定的软件硬件组合,将它们的推广性限制在其他临床环境和设备上[93,,,,94,,,,95]。
增强软组织损伤诊断
因创伤和变性引起的半月板眼泪经常引起患者的膝盖疼痛[96]。准确的诊断和适当的治疗对于改善患者的生活质量至关重要[97]。Bien等人提出的CNN模型。[98对于ACL和半月板撕裂检测,在内部验证数据集上分别达到了0.965和0.847的AUC。但是,仅使用一个MR图像评估该模型,从而限制了其临床应用。为了解决这些局限性,Fritz等人。[99] conducted a clinical validation study of a fully automated DCNN for detecting surgically confirmed meniscal tears.Compared with surgical findings and assessments by musculoskeletal radiologists, the DCNN model demonstrated fully automated detection of meniscal tears, achieving similar specificity but reduced sensitivity.Pedoia et al.[100] used a CNN for the diagnosis of meniscal injuries.They achieved 80% accuracy and reported a tendency for the misdiagnosis of posterior horn meniscal tears.
Among sport-related musculoskeletal injuries, ACL tears are common but challenging to diagnose [101,,,,102]。Researchers have developed various diagnostic models to aid in clinical analysis.The groundbreaking study by Å tajduhar et al.employed a semiautomated method using MRI data to detect ACL injuries [103]。The research utilized histograms of oriented gradients (HOGs) and global image statistics methods for feature extraction, combined with support vector machines (SVMs) and random forests, and achieved an AUC of 0.943 in detecting ACL injuries, including mild and complete ruptures.Richardson et al.[104] demonstrated that CNNs can effectively replace human readers in MRI protocol optimization, particularly in detecting ACL tears, where fat-saturated sequences outperform non-fat-saturated sequences in terms of sensitivity.To enhance generalizability, Tran et al.[105] trained the algorithm using a multicenter dataset consisting of 19,765 knee MRI scans from 12 centers, incorporating diverse scanner types (1/1.5/3Â T) and imaging protocols, demonstrating high performance across external datasets.
In addition to MR image-based diagnosis, many studies have established diagnostic models based on other criteria.刘等。[106] utilized arthroscopy as a reference standard, Zeng et al.[107] explored gait analysis to train their model, and Li et al.[108] based their approach on plantar pressure monitoring.These alternative methods expand the potential applications of AI beyond traditional imaging and contribute to the development of comprehensive, multimodal diagnostic strategies.
Although some models are trained on large and diverse datasets, their performance is often evaluated on the basis of data from single institutions, raising concerns about generalizability.Notably, improved diagnostic accuracy following retraining on external datasets suggests that model adaptation to local imaging protocols and population characteristics may be necessary [98]。These limitations underscore the need for larger, multi-institutional studies with standardized reference standards and broader clinical validation to ensure reliable real-world performance of AI models in musculoskeletal imaging.
Improving OA diagnosis and grading
In 2020, OA accounted for 4.3% of the number of years lived with disability worldwide, reflecting a 9.5% increase since 1990 and underscoring its growing impact on public health worldwide [109]。Therefore, it is particularly important to automate the detection of OA via AI.Conrozier et al.[110] introduced a method to diagnose early OA by measuring the joint gap width.The disadvantage of this method is that it requires frequent interventions by the observer to select the region of interest and adjust the bone edge detection.Unlike manual methods, which heavily depend on observer input to select regions of interest and adjust bone edge detection, this AI-driven algorithm provides an automated and objective measurement of joint space width, reducing user dependency and improving reproducibility [111]。Ãœreten et al.[112] developed a CNN-based computer-aided diagnostic method using transfer learning for hip OA detection on plain pelvic radiographs.Their model achieved 90.2% accuracy, 97.6% sensitivity, and 83.0% specificity, demonstrating the potential to assist clinicians with objective interpretation and reduce the need for advanced imaging.
Recent developments have extended AI applications to OA severity grading.To automate the evaluation of knee OA severity, Tiulpin et al.[113] introduced a DL framework utilizing CNN aligned with the Kellgren-Lawrence classification system.Their approach yielded a multiclass accuracy of 66.71%, a Cohen’s kappa (quadratic) value of 0.83, and an AUC of 0.93, indicating substantial concordance with specialist-provided labels.Similarly, another automatic Kellgren-Lawrence grading model using the DenseNet neural network architecture achieved sensitivities from 68.9 to 86.0% and specificities ranging from 83.8 to 99.1% across different severity levels, indicating strong classwise discriminatory ability [114]。A DenseNet-based model was proposed by Pedoia et al.[115] for early-stage knee OA detection, employing T2-weighted MRI imaging to facilitate diagnosis before conventional radiographic signs are evident.When patient demographic data were integrated, their model exhibited 76.99% sensitivity and 77.94% specificity across a vast patient cohort from the OA Initiative baseline dataset.These studies highlight the utility of ML techniques, particularly DL-based approaches, in automating the detection and severity grading of OA from medical imaging data.These advancements hold promise for facilitating early diagnosis, personalized treatment strategies, and improved clinical management of patients with OA.
AI-based advances in orthopedic treatment
Enhancing orthopedic surgery quality
The application of AI in orthopedic surgery has transformed the quality of care by improving precision, enhancing surgical planning, and streamlining intraoperative workflows [116,,,,117,,,,118,,,,119,,,,120]。Robotic platforms, navigation technologies, and AI-powered imaging analysis have become integral to optimize patient-specific interventions (Table 3) [45,,,,121,,,,122,,,,123,,,,124,,,,125,,,,126,,,,127,,,,128]。
Lower limb arthroplasty
AI has significantly advanced total hip arthroplasty (THA) and total knee arthroplasty (TKA) by improving preoperative planning, implant selection, and surgical precision.Chen et al.[121] investigated the relationship between the typology of DDH cases and the internal and external diameters of the socket via a 3D hip surgery simulation system, which demonstrated superior accuracy in prosthesis implantation compared with conventional two-dimensional (2D) templates.This transition from 2D to AI-enhanced 3D simulations allows for patient-specific solutions in complex developmental DDH cases, reducing errors in prosthesis placement.
For THA, AI-based tools have optimized implant recognition and alignment.Borjali et al.[45] developed a CNN-based algorithm which was evaluated on an independent test set of 252 anteroposterior hip radiographs and achieved 100% accuracy in identifying 3 common THR implant designs.These controlled test conditions demonstrate the model’s strong performance of the model, although further validation in larger clinical datasets is needed.Robot-assisted techniques have led to notable improvements in TKA, particularly in achieving more accurate mechanical alignment, minimizing intraoperative trauma, and enhancing early postoperative recovery.Systems such as Zimmer Biomet’s Robot of Stereotactic Assistant (ROSA)® and Stryker’s Mako® systems provide real-time haptic feedback during bone cutting and soft tissue balancing, enhancing intraoperative decision-making.Kayani et al.[125] confirmed that robotic arm-assisted TKA leads to significantly reduced surgical trauma compared with conventional jig-based TKA.Specifically, patients in the robotic group experienced lower postoperative pain and analgesia requirements and smaller reductions in postoperative hemoglobin levels, suggesting less intraoperative blood loss and faster functional recovery, including a shorter time to straight leg raise and earlier discharge.Rossi et al.[126] confirmed that robotic-assisted TKA improved mechanical alignment and range of motion in patients with severe varus and valgus deformities, achieving a 100% short-term survival rate with no major complications at a minimum follow-up of 6 months.Collectively, these advancements highlight the critical roles of AI and robotics in THA and TKA.AI tools aid in preoperative planning, ensuring accurate implant selection and alignment, whereas robotic systems provide real-time intraoperative guidance, improving precision and minimizing complications.
However, despite the significant role of AI and robotics in joint replacement, current research still has limitations.Limited sample size and few complex cases reduce model generalizability and recognition accuracy [45,,,,121]。Multicenter and multioperator involvement introduce variability, affecting measurement standardization [121,,,,125]。Lack of blinding, absence of correlation between short- and long-term outcomes, and inconsistent anesthesia and rehabilitation protocols restrict generalizability [125]。Future research should expand samples, standardize procedures, incorporate multimodal imaging, and strengthen long-term follow-up to increase the clinical value of these technologies.
Shoulder arthroplasty
The integration of navigation systems and robotic platforms in reverse shoulder arthroplasty has improved the accuracy of glenoid component placement, reducing manual errors such as improper inclination and off-center positioning.Giorgini et al.[124] demonstrated that computer-assisted navigation significantly improves the accuracy of glenoid component placement, reducing the number of errors associated with manual techniques.However, as Twomey-Kozak et al.[129] noted, despite these promising findings, the application of navigation systems in shoulder arthroplasty remains in its early stages, especially compared with their established use in hip and knee replacements.Darwood et al.[122] emphasized the benefits of robotic platforms for the real-time manufacturing of patient-specific instruments, reporting that the system achieved version and inclination angle accuracies of 1.9° [standard deviation (SD) 1.3] and 1.2° (SD 0.7), respectively, along with a positional accuracy of 1.1 mm (SD 0.7) relative to the preoperative plan.
Although these innovations hold promise, shoulder arthroplasty faces unique challenges that impact the effectiveness of AI-based navigation.Giorgini et al.[124] highlighted that humeral component navigation is still underdeveloped, limiting the ability to achieve optimal joint stability.Surgeons must manually align the humeral implant, which introduces variability in surgical precision.Additionally, the initial adoption of AI-based navigation systems increases the operative time, as surgeons must adapt to new workflows.Future research should aim to expand navigation capabilities to both the humeral and glenoid components, optimizing joint biomechanics and implant longevity.The integration of augmented reality and virtual reality for intraoperative guidance could enhance real-time surgical precision and efficiency [129]。
Fracture reduction
Achieving excellent reduction is crucial for fracture healing.Ankle trauma is highly prevalent, with an estimated 2 million acute sprains occurring annually in the United States [130]。Due to the complexity of the anatomy, even minor injuries can lead to serious consequences [131], and up to 70% of patients may experience residual physical disability.Improper reduction of the joint during surgery can result in an insufficient contact area or excessive contact force, thereby increasing the risk of postoperative complications [132,,,,133]。AI-based fracture reduction has been developed to minimize intraoperative fluoroscopy exposure while improving reduction accuracy.Gebremeskel et al.[123] proposed an image-guided robotic system for ankle fracture reduction, utilizing the contralateral ankle as a reference to determine optimal manipulative forces and displacement parameters.This study quantified the manipulative forces and their displacement necessary to reduce the ankle symphysis accurately, marking a crucial first step in defining the design requirements for robotic assistance in terms of force and displacement.Robotic-assisted fracture reduction has notable limitations.The effectiveness of AI-based force prediction models is dependent on the variability of individual patient anatomy, and real-time adaptation remains a challenge.Additionally, current robotic reduction techniques lack intraoperative feedback mechanisms, which may lead to unintended overcorrection or soft tissue complications.Future efforts should focus on real-time AI adjustments using intraoperative fluoroscopic or CT-based feedback systems to ensure optimal reduction without excessive manual interventions.
Ligament reconstruction
AI has improved ligament reconstruction procedures by enhancing surgical planning and intraoperative precision.Sakakibara et al.[127] introduced a robotic biomechanical testing system for simulating joint kinematics and tendon graft placement, suggesting reconstruction at 30° of plantar flexion for optimal outcomes.Additionally, Yang et al.[128] utilized AI-based 3D CT and MRI analysis for personalized ACL reconstruction planning, integrating robotic assistance for real-time surgical adjustments.Their study demonstrated that robotic guidance improved bone tunnel drilling accuracy, maintaining deviations within 1.5 mm.
Accelerating drug development
New drug development is a long and complex process characterized by high R&D costs and significant uncertainty [134]。In recent years, traditional methods of new drug R&D have become increasingly difficult, with increasing investments and extended development times.The field is currently at a bottleneck stage, necessitating new technologies to achieve cost reduction and increased efficiency.The rapid advancement of AI technology has introduced new opportunities for high-quality developments in the biopharmaceutical industry [135]。As early as the 1980s, Merck began to design drugs using computer-aided design [136]。With the progression of computer technology, AI has gradually predominated, becoming increasingly involved in the drug development process [137]。This approach ensures the quality of analysis while significantly reducing drug R&D costs, shortening development time, and improving overall efficiency, thereby putting new drug development on a fast and efficient path [138,,,,139]。AI has found applications in predicting protein structure and functional properties [46,,,,47,,,,48,,,,140,,,,141,,,,142], forecasting drug‒protein interactions [143,,,,144], and facilitating high-throughput drug screening [145]。
Predicting protein 3D structures, properties, and functions
Understanding the 3D structures, properties, and functions of proteins is a critical step in drug discovery and development.An important example of such efforts is the study by Jumper et al.[46], who developed AlphaFold, a neural network model that provides a computational method for predicting protein structures with atomic accuracy, even in the absence of a known analogous structure.The results showed that, in most cases, its accuracy is comparable to that of the experimental structures and greatly outperforms that of the other methods.
In 2023, Yuan et al.[142] proposed a DL framework that combines bidirectional temporal convolutional networks, bidirectional long short-term memory, and a multiscale bidirectional temporal convolutional network for secondary protein structure prediction, achieving superior performance over existing methods on the Critical Assessment of protein Structure Prediction 10 – 14 (CASP10 – 14) and CullPDB 513 (CB513) benchmark datasets.Wang et al.[48] developed a DL framework, a language model with a geometric vector perceptron, consisting of a protein language model and a graph neural network, which can make predictions about protein properties by utilizing one-dimensional amino acid sequences and 3D structural information of proteins.Gligorijević et al.[140] introduced deep functional residue identification (DeepFRI), a graphical convolutional network that outperforms current major methods and sequence-based CNNs.DeepFRI uses sequence features extracted from protein language models and protein structures to predict protein functions.AI technology has great potential for accurately predicting protein structures and functions, which will lead to unprecedented technological innovations for new drug development.
Predicting drug-protein interactions
Identifying the target proteins of a drug and predicting the drug-target protein interactions play extremely important roles in the drug development process.By predicting drug-receptor or -protein interactions, researchers can better understand the efficacy of a drug and design it most effectively.For example, Offensperger et al.[143] discovered hundreds of interactions between fragments and proteins through a large-scale chemical proteomics survey.They reported that the data generated are suitable for ML-based models, which, when the chemical structure is used as input, can predict how the chemical interacts with the native proteome in intact cells.Investigating protein–ligand interactions, Wang et al.[144] trained an SVM model on 15,000 ligand–protein interactions involving 626 proteins and 10,000 active compounds.This model successfully identified 9 new compounds and their interactions with 4 key targets (G protein-coupled receptor 4, sirtuin 1, p38 mitogen-activated protein kinase, and glycogen synthase kinase 3 beta).Predicting drug-protein interactions will accelerate the drug development process by reducing the time required for target screening.
High-throughput drug screening
Although AI has not yet been widely applied to screen drug targets for orthopedic diseases, its notable success in other disease areas has driven a growing number of companies and large pharmaceutical firms to invest in AI-based drug discovery efforts [135]。The PandaOmics platform exemplifies how multiple AI engines can accelerate drug development by identifying promising targets [145]。In the case of INS018_055 [145], PandaOmics integrated multiomics data, literature trends, and biological network analysis to identify TNIK as a novel therapeutic target within just 18Â months.
Although AI technology provides unprecedented opportunities for the discovery and development of novel orthopedic drugs by significantly improving the efficiency of drug screening and optimization, several challenges remain.First, in terms of data, there is a notable scarcity of high-quality, consistent, and accessible datasets [137,,,,146]。Unlike in fields such as image recognition, drug discovery suffers from limited annotated experimental data due to the inherent complexity of biological systems and variability in experimental conditions, leading to inconsistent and unreliable results.Second, challenges with molecular representation persist.Model performance is highly sensitive to the type and quality of molecular input used.Most current representations fail to account for essential aspects such as stereochemistry, conformational flexibility, the molecular surface, and compactness, all of which are crucial to molecular function [146]。Moreover, selecting an appropriate representation often involves a trade-off between simplicity and expressiveness, complicating the goal of model interpretability [137]。To fully realize the potential of AI, a collaborative effort is necessary to improve data quality, increase model transparency, and foster interdisciplinary collaboration.
Contributing to intelligent rehabilitation
Surgery is not the endpoint of orthopedic disease treatment.Postoperative complications such as joint stiffness and ossification can severely affect patient prognosis, making rehabilitation a crucial part of orthopedic postoperative care.With advancements in AI R&D, rehabilitation robots and other AI-based technologies are playing a significant role in assisting in rehabilitative treatment.
Recent advancements in intelligent rehabilitation systems have demonstrated the potential of AI to support complex motor function recovery.Averta et al.[147] designed a novel human-like motion generation algorithm that analyzes human motion through functional principal component analysis features, enabling efficient synthesis of complex movements such as free motion and obstacle avoidance in free space.Building upon the concept of human motion analysis, Zhao et al.[148] applied these principles to rehabilitation by designing a tele-rehabilitation system that integrates natural human responses with big data analytics.Their system includes an upper limb rehabilitation robot that combines flexible ropes and exoskeleton components and is intended to assist clinicians in optimizing individualized rehabilitation programs.To further enhance rehabilitation management, they proposed a multidimensional training and assessment database to support a multilevel linked rehabilitation system.Extending robotic applications to lower extremity rehabilitation, Miller-Jackson et al.[149] designed a soft pneumatic actuator-driven exoskeleton for hip flexor rehabilitation, providing more options for individuals with lower extremity mobility issues.Compared with not wearing the device, subjects experienced a 43.5% reduction in muscle signals when lifting their legs while wearing the device, suggesting that the device is effective in assisting with hip flexion and that a pneumatic rotary actuator-driven exoskeleton is a viable solution.In conjunction with these hardware-based solutions, digital platforms such as smartphone-based applications are also playing an increasingly important role in rehabilitation.Rossi et al.[150] investigated the use of a smartphone-based care management platform, myMobility, in the context of TKA rehabilitation.
Personalized treatment planning
Precision medicine aims to tailor treatment strategies by classifying patients into subgroups based on variations in prognosis or treatment response, thereby optimizing therapeutic benefits while minimizing unnecessary interventions [151]。AI algorithms are well equipped to handle high-dimensional data, support predictive modeling, and inform personalized management strategies in clinical settings [152]。AI is transforming surgical planning and clinical decision-making by analyzing patient-specific data to predict outcomes, optimize procedures, and guide personalized strategies (Table 4) [32,,,,33,,,,34,,,,35,,,,36,,,,49,,,,153,,,,154,,,,155,,,,156,,,,157,,,,158,,,,159,,,,160]。
Predictive analytical models have been applied extensively in adult spinal deformity surgeries.For example, Ames et al.[32] used an ML model to predict functional outcome and quality of life after instrumental correction of adult spinal deformity.This model takes multiple factors into account, tailoring predictions to the unique circumstances of each patient, thereby improving preoperative counseling and decision-making.Building on this work, the same team later introduced a refined classification framework based on hierarchical clustering [153], which enhances decision-making by identifying data patterns.This system offers 2-year risk‒benefit grids, guiding surgeons in selecting the most appropriate strategies, such as posterior fusion, intervertebral implantation, or pedicle subtraction osteotomy.
Model interpretability plays a crucial role in clinical adoption.Transparent models allow clinicians to understand key predictive factors, such as age, comorbidities, and surgical complexity, ensuring alignment with clinical reasoning.This fosters trust between patients and providers and mitigates potential biases, improving the generalizability of models across diverse populations.Interpretability also enhances personalized treatment planning, as demonstrated by Pellisé et al.[157]。The classification and regression tree algorithm was used to predict complications such as nonunion, vertebral kyphosis, and pedicle screw loosening, providing preoperative insights into the risk of implant failure and guiding individualized interventions.Similarly, Cattaneo et al.[155] applied factorial analysis to study gait changes post-THA, demonstrating how different surgical approaches, i.e., posterior vs. anterior, impact recovery trajectories.These findings emphasize that AI-powered predictive models align surgical strategies with individual patient needs, enhancing postoperative outcomes.
AI is likewise used for postsurgical evaluation and prediction of complications after other procedures, such as THA, arthroscopic rotator cuff repair (ARCR), ankle fracture open reduction internal fixation (ORIF), TKA, and flushing and debridement after periprosthetic joint infection (PJI).To predict opioid use after THA, Karhade et al.[33] designed a preoperative ML algorithm that analyzed a total of 5507 patients.The study revealed that 345 patients had long-term postoperative opioid use and identified predictors of long-term opioid prescription.The best model had an AUC of 0.77, indicating high net returns.Potty et al.[36] proposed a new ML algorithm to predict the American Shoulder and Elbow Surgeons score after ARCR with relatively high accuracy.Merrill et al.[35] used ML to predict short-term outcomes after ORIF for ankle fractures.ML accurately predicted several comorbidities associated with poor short-term outcomes after ORIF for ankle fractures.Klemt et al.[34] developed and validated 3 ML models to predict the recurrence of PJI after TKA.The factors related to recurrence after TKA were irrigation and debridement, > 4 previous open surgeries, metastatic disease, drug abuse, acquired immune deficiency syndrome, enterococcal infection, and obesity.All the ML models achieved good discrimination performance.Shohat et al.[158] designed a retrospective study that collected data on 1174 THA and TKA procedures.They used a randomized forest ML model in assessing 52 variables to predict outcomes after irrigation and debridement for PJI.The model achieved good discrimination (AUC = 0.74) and high accuracy.In addition, the model identified 10 significant factors associated with failure, such as positive blood cultures and elevated C-reactive protein levels.
AI has gradually transformed sports medicine, especially in the area of ACL injury risk prediction.AI methods have facilitated the identification of biomechanical factors associated with ACL injury risk, supporting subsequent developments in imaging-based and sensor-driven predictive models [156,,,,159,,,,160,,,,161]。Pedoia et al.[161] used 3D MRI-based statistical shape modeling to uncover anatomical features associated with ACL injury, such as specific intercondylar width and posterior tibial slope values.Johnson et al.[156] developed a DL-based system that uses a pretrained CNN to monitor knee joint movements in real time.Movement during activities such as walking, running, and sidestepping is analyzed to detect ACL injury risk, offering valuable insights for athletic training and injury prevention.Taborri et al.[159] quantified ACL injury risk by assessing stability and load absorption through inertial sensors and optoelectronic devices.Research has demonstrated that the landing error score system strongly correlates with the risk of ACL injury.Tamimi et al.[160] built a supervised ML-based predictive model using knee morphology data from MRI scans to predict primary ACL injury, achieving 92% testing accuracy and highlighting the role of AI in structural analysis and early intervention.
AI has also demonstrated value in predicting the progression of chronic diseases.In a recent study, Leung et al.[49] developed a DL prediction model for OA progression risk that could directly predict the need for TKA based on knee radiographs.Compared with the binary outcome model using a standard grading system, the proposed DL model better predicted the risk of TKA for OA.Bertsimas et al.[154] demonstrated the value of ML in improving clinical decision-making in pediatric trauma patients.They retrospectively assessed the risk of cervical spine injury in pediatric patients in conjunction with an optimal classification tree algorithm, achieving a sensitivity of 93.3% and a specificity of 82.3%.These models enable health care professionals to make informed, data-driven decisions, ensuring timely interventions and optimal patient outcomes.
By facilitating the early detection of potential risk factors, interpretable computational models contribute to the development of individualized preventive strategies and may assist in improving clinical decision-making.Within orthopedic practice, such models can aid in tailoring treatment plans, refining preoperative assessments, and supporting postoperative management.In areas such as complication risk assessment, rehabilitation planning, and injury prevention, data-driven approaches offer valuable support for predictive analyses.The capacity to process complex clinical data, recognize subtle associations, and provide interpretable outputs underscores the potential utility of these tools in the context of orthopedics and sports medicine.
Limitations and future perspectives
Progress in AI applications for musculoskeletal imaging and orthopedic care has been substantial;however, numerous limitations persist that affect the reliability and integration of AI-based methods into clinical practice.
Limitations in data and model design
The training of AI models heavily depends on large-scale, high-quality, and diverse datasets.However, in orthopedics, data scarcity remains a major issue [162]。Most current studies have relied on imaging data from single centers, specific equipment, and defined populations, which limits the representativeness and generalizability of the results [40,,,,56,,,,163,,,,164]。Additionally, the annotation process involves subjectivity and lacks uniform standards [41,,,,56];for example, the use of radiology reports or manual segmentation as reference standards introduces potential bias into the model.
The inherent “black box†nature of AI systems further increases uncertainty in clinical applications [165]。Although deep models can achieve high predictive accuracy, they usually lack interpretability and fail to provide clinicians with understandable decision paths, especially in complex clinical scenarios.Although post hoc visualization methods can assist with partial interpretation, they do not fully replace traceable clinical logic [162]。Furthermore, AI models are vulnerable to adversarial perturbations [166], where small modifications to input images may result in incorrect predictions, posing risks to model safety and reliability.
Technical and operational challenges in clinical application
The clinical application of AI is currently limited by various technical and operational challenges.First, most AI systems are designed for specific tasks such as image classification or structure recognition, and are unable to address broader clinical workflows, including disease correlation and individualized treatment planning [66,,,,85]。Moreover, AI performs poorly in subjective assessments and cannot handle ambiguous or uncertain information [92], which further limits its utility in clinical scenarios.Second, AI systems are highly dependent on data and computational resources.In practice, many models require significant computing power and specific software-hardware configurations [93,,,,162], making deployment in resource-limited settings difficult.Model performance is also affected by image quality and the presence of anatomical landmarks, and variations in imaging protocols between hospitals add further complexity to deployment [93]。
From a translational perspective, clinical validation remains insufficient.Many studies are based on data from single institutions, and model generalizability across different regions, equipment, and populations has not been adequately tested.For example, only 11% of fracture detection studies reported external geographical validation [167], significantly limiting the clinical applicability of the results.Adaptability to specific populations and complex cases also remains a critical concern.Current AI systems have yet to receive FDA approval for pediatric fracture detection [85], as pediatric fractures often exhibit unique radiographic features that necessitate specialized detection algorithms [168]。Additionally, the performance of AI models in detecting stress fractures, pathological fractures, and abnormalities in bone structure still requires improvement [121]。Beyond these challenges, the lack of long-term follow-up data and limited validation of real-world clinical outcomes hinder the translation of AI-based methods from research settings to routine orthopedic practice.Short-term diagnostic accuracy does not necessarily equate to long-term therapeutic benefits, and few studies have established definitive links between AI-assisted interventions and patient prognoses over time [41,,,,125]。
Therefore, to achieve effective translation and clinical adoption of AI-based methods in orthopedics, it is necessary to optimize deployment processes;enhance model functionality, strengthen multicenter, multimodal, large-scale clinical validation;and promote real-world research design to improve adaptability and reliability in complex clinical environments.
Acceptance, cost, and ethical considerations
AI still faces considerable barriers to clinical acceptance.One major issue is the lack of clinician trust in “black box†decision-making systems, especially in critical diagnostic scenarios where the reasoning path is not transparent [169,,,,170]。Additionally, health care providers, particularly less experienced interns or junior doctors, may become overly reliant on AI systems, which could impair the development of their independent clinical judgment [66]。
Another significant challenge involves ethical and legal regulation [171]。At present, there is no unified standard for obtaining informed consent for the use of data in model training, and issues such as algorithmic fairness, justice, and data privacy lack systematic evaluation.As developers are not medical professionals, their legal responsibility remains undefined, and clearer regulatory guidance and stakeholder negotiation are urgently needed.Furthermore, AI models often inherit biases from training data, which may amplify systemic inequities related to race or gender.To date, most studies have not considered population diversity or ethnic differences [172]。
未来的观点
In summary, although AI methods are developing rapidly and extensively in orthopedics, their clinical application is still limited by several factors, including data quality and scale, model stability, the complexity of clinical environments, medical trust, and regulatory mechanisms.To advance the field, future research should focus on the following aspects: establishing high-quality, multicenter, ethnically diverse data platforms to promote standardized data sharing;developing more interpretable model architectures to increase clinical trust;expanding AI capabilities to cover the full diagnostic and treatment chain, including disease progression prediction, surgical planning, and long-term outcome evaluation;strengthening ethical review and regulatory frameworks to clarify the responsibilities of developers and users;and promoting long-term, multiregional, real-world clinical validation and iterative optimization.Only by addressing these challenges can AI in orthopedics truly evolve from a “laboratory technology†to a “clinical productivity toolâ€.
结论
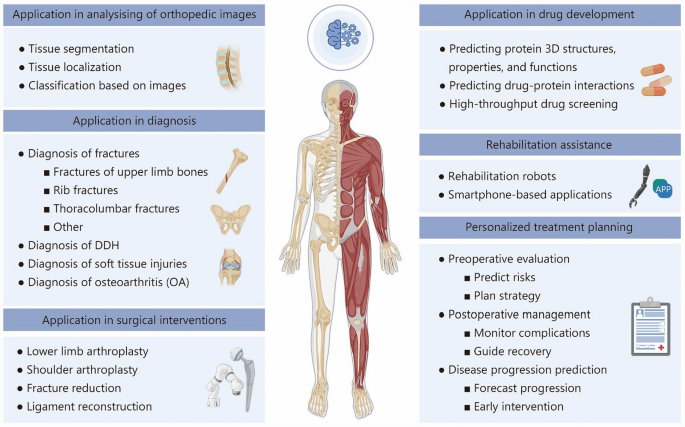
This review provides a comprehensive overview of the current applications of computational technologies in orthopedics (Fig. 3), with a particular focus on their roles in imaging analysis, clinical diagnosis, and various aspects of treatment, including surgical planning, drug development, rehabilitation, and personalized care.These tools have been employed to enhance the precision and efficiency of image interpretation, support clinical decision-making, and contribute to individualized therapeutic strategies.At the same time, this review also identifies a range of limitations that continue to hinder their broader implementation.These include constraints related to data availability and model generalizability, challenges in integrating such tools into complex clinical workflows, and concerns surrounding ethical oversight, regulatory standards, and long-term clinical validation.Future research should prioritize the development of interpretable and robust systems, the construction of diverse and high-quality datasets, and the establishment of multidisciplinary frameworks to ensure the responsible and effective incorporation of these technologies into orthopedic practice.
数据和材料的可用性
Not applicable.
缩写
- 2D:
-
Two-dimensional
- 3D:
-
三维
- ACL:
-
Anterior cruciate ligament
- 人工智能:
-
人工智能
- ARCR:
-
Arthroscopic rotator cuff repair
- AUC:
-
Area under the curve
- CASP:
-
Critical assessment of protein structure prediction
- CB513:
-
CullPDB 513
- CBCT:
-
Cone-beam computed tomography
- CNN:
-
Convolutional neural networks
- CT:
-
Computed tomography
- DCNN:
-
Deep convolutional neural network
- DDH:
-
Developmental dysplasia of the hip
- DeepFRI:
-
Deep functional residue identification
- DL:
-
Deep learning
- DNN:
-
Deep neural networks
- HOG:
-
Histogram of oriented gradient
- IHDI grade:
-
International Hip Dysplasia Institute grade
- ML:
-
机器学习
- 先生:
-
Magnetic resonance
- MRI:
-
Magnetic resonance imaging
- MURA:
-
Musculoskeletal radiograph
- OA:
-
Osteoarthritis
- ORIF:
-
Open reduction internal fixation
- PHOG:
-
Pyramidal histogram of oriented gradient
- PJI:
-
Periprosthetic joint infection
- R&D:
-
研发
- SD:
-
Standard deviation
- SVM:
-
Support vector machine
- THA:
-
Total hip arthroplasty
- TKA:
-
Total knee arthroplasty
参考
Rajpurkar P, Chen E, Banerjee O, Topol EJ.AI in health and medicine.Nat Med。2022;28(1):31–8.
CAS一个 Google Scholar一个
Makary MA, Daniel M. Medical error—the third leading cause of death in the US.BMJ。2016;353:i2139.
Xiang Y, Zhao L, Liu Z, Wu X, Chen J, Long E, et al.Implementation of artificial intelligence in medicine: status analysis and development suggestions.Artif Intell Med.2020;102:101780.
Malik AT, Khan SN.Predictive modeling in spine surgery.Ann Transl Med.2019;7(Suppl 5):S173.
Wu E, Wu K, Daneshjou R, Ouyang D, Ho DE, Zou J. How medical AI devices are evaluated: limitations and recommendations from an analysis of FDA approvals.Nat Med。2021;27(4):582–4.
CAS一个 Google Scholar一个
Hui AT, Alvandi LM, Eleswarapu AS, Fornari ED.Artificial intelligence in modern orthopaedics: current and future applications.JBJS Rev. 2022.https://doi.org/10.2106/JBJS.RVW.22.00086。Google Scholar
一个 Charles YP, Lamas V, Ntilikina Y. Artificial intelligence and treatment algorithms in spine surgery.
Orthop Traumatol Surg Res.2023;109(1S):103456.
Gyftopoulos S, Lin D, Knoll F, Doshi AM, Rodrigues TC, Recht MP.Artificial intelligence in musculoskeletal imaging: current status and future directions.AJR Am J Roentgenol.2019;213(3):506–13.
Ho D, Quake SR, McCabe ERB, Chng WJ, Chow EK, Ding X, et al.Enabling technologies for personalized and precision medicine.Trends Biotechnol.2020;38(5):497–518.
CAS一个 Google Scholar一个
Patel AA, Schwab JH, Amanatullah DF, Divi SN.AOA critical issues symposium: shaping the impact of artificial intelligence within orthopaedic surgery.J Bone Joint Surg Am.2023;105(18):1475–9.
Cheng K, Guo Q, He Y, Lu Y, Xie R, Li C, et al.Artificial intelligence in sports medicine: could GPT-4 make human doctors obsolete?Ann Biomed Eng.2023;51(8):1658–62.
Cabitza F, Locoro A, Banfi G. Machine learning in orthopedics: a literature review.Front Bioeng Biotechnol.2018;6:75.
Haug CJ, Drazen JM.Artificial intelligence and machine learning in clinical medicine, 2023. N Engl J Med.2023;388(13):1201–8.
CAS一个 Google Scholar一个
Mintz Y, Brodie R. Introduction to artificial intelligence in medicine.Minim Invasive Ther Allied Technol.2019;28(2):73–81.
Li Z, Song P, Li G, Han Y, Ren X, Bai L, et al.AI energized hydrogel design, optimization and application in biomedicine.Mater Today Bio.2024;25:101014.
CAS一个 Google Scholar一个
Chang M, Canseco JA, Nicholson KJ, Patel N, Vaccaro AR.The role of machine learning in spine surgery: the future is mow.Front Surg.2020;7:54.
Chang TC, Seufert C, Eminaga O, Shkolyar E, Hu JC, Liao JC.Current trends in artificial intelligence application for endourology and robotic surgery.Urol Clin North Am.2021;48(1):151–60.
LeCun Y, Bengio Y, Hinton G. Deep learning.自然。2015;521(7553):436–44.
CAS一个 Google Scholar一个
Kaul V, Enslin S, Gross SA.History of artificial intelligence in medicine.Gastrointest Endosc.2020;92(4):807–12.
Zhang YP, Zhang XY, Cheng YT, Li B, Teng XZ, Zhang J, et al.Artificial intelligence-driven radiomics study in cancer: the role of feature engineering and modeling.Mil Med Res。2023;10(1):22.
Zeng S, Wang XL, Yang H. Radiomics and radiogenomics: extracting more information from medical images for the diagnosis and prognostic prediction of ovarian cancer.Mil Med Res。2024;11(1):77.
CAS一个 Google Scholar一个
Ramkumar PN, Luu BC, Haeberle HS, Karnuta JM, Nwachukwu BU, Williams RJ.Sports medicine and artificial intelligence: a primer.Am J Sports Med.2022;50(4):1166–74.
Avanzo M, Stancanello J, Pirrone G, Drigo A, Retico A. The evolution of artificial intelligence in medical imaging: from computer science to machine and deep learning.癌症(巴塞尔)。2024;16(21):3702.
CAS一个 Google Scholar一个
Bai L, Wu Y, Li G, Zhang W, Zhang H, Su J. AI-enabled organoids: construction, analysis, and application.Bioact Mater.2024;31:525–48.
Myers TG, Ramkumar PN, Ricciardi BF, Urish KL, Kipper J, Ketonis C. Artificial intelligence and orthopaedics: an introduction for clinicians.J Bone Joint Surg Am.2020;102(9):830–40.
Deo RC.Machine learning in medicine.循环。2015;132(20):1920–30.
Galbusera F, Casaroli G, Bassani T. Artificial intelligence and machine learning in spine research.JOR Spine.2019;2(1):e1044.
Chafai N, Luigi B, Sara B, Badaoui B. Emerging applications of machine learning in genomic medicine and healthcare.Crit Rev Clin Lab Sci.2024;61(2):140–63.
CAS一个 Google Scholar一个
Hassan M, Awan FM, Naz A, deAndrés-Galiana EJ, Alvarez O, Cernea A, et al.Innovations in genomics and big data analytics for personalized medicine and health care: a review.Int J Mol Sci。2022;23(9):4645.
CAS一个 Google Scholar一个
Kotti M, Duffell LD, Faisal AA, McGregor AH.Detecting knee osteoarthritis and its discriminating parameters using random forests.Med Eng Phys.2017;43:19–29.
Luu BC, Wright AL, Haeberle HS, Karnuta JM, Schickendantz MS, Makhni EC, et al.Machine learning outperforms logistic regression analysis to predict next-season NHL player injury: an analysis of 2322 players from 2007 to 2017. Orthop J Sports Med.2020;8(9):2325967120953404.
Ames CP, Smith JS, Pellisé F, Kelly M, Gum JL, Alanay A, et al.Development of predictive models for all individual questions of SRS-22R after adult spinal deformity surgery: a step toward individualized medicine.Eur Spine J. 2019;28(9):1998–2011.
Karhade AV, Schwab JH, Bedair HS.Development of machine learning algorithms for prediction of sustained postoperative opioid prescriptions after total hip arthroplasty.J Arthroplast.2019;34(10):2272-7.e1.
Klemt C, Laurencin S, Uzosike AC, Burns JC, Costales TG, Yeo I, et al.Machine learning models accurately predict recurrent infection following revision total knee arthroplasty for periprosthetic joint infection.Knee Surg Sports Traumatol Arthrosc.2022;30(8):2582–90.
Merrill RK, Ferrandino RM, Hoffman R, Shaffer GW, Ndu A. Machine learning accurately predicts short-term outcomes following open reduction and internal fixation of ankle fractures.J Foot Ankle Surg.2019;58(3):410–6.
Potty AG, Potty ASR, Maffulli N, Blumenschein LA, Ganta D, Mistovich RJ, et al.Approaching artificial intelligence in orthopaedics: predictive analytics and machine learning to prognosticate arthroscopic rotator cuff surgical outcomes.J Clin Med。2023;12(6):2369.
Martin RK, Wastvedt S, Pareek A, Persson A, Visnes H, Fenstad AM, et al.Unsupervised machine learning of the combined Danish and Norwegian knee ligament registers: identification of 5 distinct patient groups with differing ACL revision rates.Am J Sports Med.2024;52(4):881–91.
Tran KA, Kondrashova O, Bradley A, Williams ED, Pearson JV, Waddell N. Deep learning in cancer diagnosis, prognosis and treatment selection.Genome Med.2021;13(1):152.
Dias R, Torkamani A. Artificial intelligence in clinical and genomic diagnostics.Genome Med.2019;11(1):70.
Hemke R, Buckless CG, Tsao A, Wang B, Torriani M. Deep learning for automated segmentation of pelvic muscles, fat, and bone from CT studies for body composition assessment.Skeletal Radiol.2020;49(3):387–95.
Norman B, Pedoia V, Majumdar S. Use of 2D U-net convolutional neural networks for automated cartilage and meniscus segmentation of knee MR imaging data to determine relaxometry and morphometry.Radiology.2018;288(1):177–85.
Chung SW, Han SS, Lee JW, Oh K-S, Kim NR, Yoon JP, et al.Automated detection and classification of the proximal humerus fracture by using deep learning algorithm.Acta Orthop.2018;89(4):468–73.
Kim DH, MacKinnon T. Artificial intelligence in fracture detection: transfer learning from deep convolutional neural networks.Clin Radiol.2018;73(5):439–45.
CAS一个 Google Scholar一个
Lindsey R, Daluiski A, Chopra S, Lachapelle A, Mozer M, Sicular S, et al.Deep neural network improves fracture detection by clinicians.Proc Natl Acad Sci U S A. 2018;115(45):11591–6.
CAS一个 Google Scholar一个
Borjali A, Chen AF, Muratoglu OK, Morid MA, Varadarajan KM.Detecting total hip replacement prosthesis design on plain radiographs using deep convolutional neural network.J Orthop Res.2020;38(7):1465–71.
Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, et al.Highly accurate protein structure prediction with AlphaFold.自然。2021;596(7873):583–9.
CAS一个 Google Scholar一个
Wang S, Peng J, Ma J, Xu J. Protein secondary structure prediction using deep convolutional neural fields.Sci Rep. 2016;6(1):18962.
CAS一个 Google Scholar一个
Wang Z, Combs SA, Brand R, Calvo MR, Xu P, Price G, et al.LM-GVP: an extensible sequence and structure informed deep learning framework for protein property prediction.Sci Rep. 2022;12(1):6832.
CAS一个 Google Scholar一个
Leung K, Zhang B, Tan J, Shen Y, Geras KJ, Babb JS, et al.Prediction of total knee replacement and diagnosis of osteoarthritis by using deep learning on knee radiographs: data from the osteoarthritis initiative.Radiology.2020;296(3):584–93.
Burström G, Buerger C, Hoppenbrouwers J, Nachabe R, Lorenz C, Babic D, et al.Machine learning for automated 3-dimensional segmentation of the spine and suggested placement of pedicle screws based on intraoperative cone-beam computer tomography.J Neurosurg Spine.2019;31(1):147–54.
Chae J, Kang Y-J, Noh Y. A deep-learning approach for foot-type classification using heterogeneous pressure data.传感器。2020;20(16):4481.
Fan G, Liu H, Wu Z, Li Y, Feng C, Wang D, et al.Deep learning–based automatic segmentation of lumbosacral nerves on CT for spinal intervention: a translational study.AJNR Am J Neuroradiol.2019;40(6):1074–81.
CAS一个 Google Scholar一个
Ghidotti A, Vitali A, Regazzoni D, Cohen MW, Rizzi C. Comparative analysis of convolutional neural network architectures for automated knee segmentation in medical imaging: a performance evaluation.J Comput Inf Sci Eng.2024;24(5):051005.
Glocker B, Feulner J, Criminisi A, Haynor DR, Konukoglu E, editors.Automatic localization and identification of vertebrae in arbitrary field-of-view CT scans.Med Image Comput Comput Assist Interv.2012;15(Pt 3):590–8.
Jamaludin A, Lootus M, Kadir T, Zisserman A, Urban J, Battié MC, et al.ISSLS prize in bioengineering science 2017: automation of reading of radiological features from magnetic resonance images (MRIs) of the lumbar spine without human intervention is comparable with an expert radiologist.Eur Spine J. 2017;26(5):1374–83.
Lind A, Akbarian E, Olsson S, Nåsell H, Sköldenberg O, Razavian AS, et al.Artificial intelligence for the classification of fractures around the knee in adults according to the 2018 AO/OTA classification system.PLoS ONE.2021;16(4):e0248809.
CAS一个 Google Scholar一个
Oktay AB, Akgul YS.Simultaneous localization of lumbar vertebrae and intervertebral discs with SVM-based MRF.IEEE Trans Biomed Eng.2013;60(9):2375–83.
Shah RF, Martinez AM, Pedoia V, Majumdar S, Vail TP, Bini SA.Variation in the thickness of knee cartilage.The use of a novel machine learning algorithm for cartilage segmentation of magnetic resonance images.J Arthroplast.2019;34(10):2210–5.
Jakubicek R, Chmelik J, Jan J, Ourednicek P, Lambert L, Gavelli G. Learning-based vertebra localization and labeling in 3D CT data of possibly incomplete and pathological spines.Comput Methods Programs Biomed.2020;183:105081.
Kwan JL, Calder LA, Bowman CL, MacIntyre A, Mimeault R, Honey L, et al.Characteristics and contributing factors of diagnostic error in surgery: analysis of closed medico-legal cases and complaints in Canada.Can J Surg.2024;67(1):E58.
Federico CA, Trotsyuk AA.Biomedical data science, artificial intelligence, and ethics: navigating challenges in the face of explosive growth.Annu Rev Biomed Data Sci.2024;7(1):1–14.
Rabie AH, Saleh AI.Diseases diagnosis based on artificial intelligence and ensemble classification.Artif Intell Med.2024;148:102753.
Guermazi A, Tannoury C, Kompel AJ, Murakami AM, Ducarouge A, Gillibert A, et al.Improving radiographic fracture recognition performance and efficiency using artificial intelligence.Radiology.2022;302(3):627–36.
Link TM, Pedoia V. Using AI to improve radiographic fracture detection.Radiology.2022;302(3):637–8.
Wu AM, Bisignano C, James SL, Abady GG, Abedi A, Abu-Gharbieh E, et al.Global, regional, and national burden of bone fractures in 204 countries and territories, 1990–2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet Healthy Longev.2021;2(9):e580–92.
Chen K, Stotter C, Klestil T, Nehrer S. Artificial intelligence in orthopedic radiography analysis: a narrative review.诊断。2022;12(9):2235.
Ajmera P, Kharat A, Botchu R, Gupta H, Kulkarni V. Real-world analysis of artificial intelligence in musculoskeletal trauma.J Clin Orthop Trauma.2021;22:101573.
Shen L, Gao C, Hu S, Kang D, Zhang Z, Xia D, et al.Using artificial intelligence to diagnose osteoporotic vertebral fractures on plain radiographs.J Bone Miner Res.2023;38(9):1278–87.
Beyaz S, Açıcı K, Sümer E. Femoral neck fracture detection in X-ray images using deep learning and genetic algorithm approaches.Jt Dis Relat Surg.2020;31(2):175–83.
Burns JE, Yao J, Summers RM.Vertebral body compression fractures and bone density: automated detection and classification on CT images.Radiology.2017;284(3):788–97.
Guan B, Zhang G, Yao J, Wang X, Wang M. Arm fracture detection in X-rays based on improved deep convolutional neural network.Comput Electr Eng.2020;81:106530.
Jones RM, Sharma A, Hotchkiss R, Sperling JW, Hamburger J, Ledig C, et al.Assessment of a deep-learning system for fracture detection in musculoskeletal radiographs.NPJ Digit Med.2020;3:144.
Langerhuizen DWG, Bulstra AEJ, Janssen SJ, Ring D, Kerkhoffs GMMJ, Jaarsma RL, et al.Is deep learning on par with human observers for detection of radiographically visible and occult fractures of the scaphoid?Clin Orthop Relat Res。2020;478(11):2653–9.
Li YC, Chen HH, Horng-Shing LuH, Hondar Wu HT, Chang MC, Chou PH.Can a deep-learning model for the automated detection of vertebral fractures approach the performance level of human subspecialists?Clin Orthop Relat Res。2021;479(7):1598–612.
Niiya A, Murakami K, Kobayashi R, Sekimoto A, Saeki M, Toyofuku K, et al.Development of an artificial intelligence-assisted computed tomography diagnosis technology for rib fracture and evaluation of its clinical usefulness.Sci Rep. 2022;12(1):8363.
CAS一个 Google Scholar一个
Pranata YD, Wang KC, Wang JC, Idram I, Lai JY, Liu JW, et al.Deep learning and SURF for automated classification and detection of calcaneus fractures in CT images.Comput Methods Programs Biomed.2019;171:27–37.
Sato Y, Takegami Y, Asamoto T, Ono Y, Hidetoshi T, Goto R, et al.Artificial intelligence improves the accuracy of residents in the diagnosis of hip fractures: a multicenter study.BMC Musculoskelet Disord.2021;22(1):407.
Yao L, Guan X, Song X, Tan Y, Wang C, Jin C, et al.Rib fracture detection system based on deep learning.Sci Rep. 2021;11(1):23513.
CAS一个 Google Scholar一个
Wu J, Liu N, Li X, Fan Q, Li Z, Shang J, et al.Convolutional neural network for detecting rib fractures on chest radiographs: a feasibility study.BMC Med Imaging.2023;23(1):18.
CAS一个 Google Scholar一个
Briody H, Hanneman K, Patlas MN.Applications of artificial intelligence in acute thoracic imaging.Can Assoc Radiol J. 2025.https://doi.org/10.1177/08465371251322705。Google Scholar
一个 Rosenberg GS, Cina A, Schiró GR, Giorgi PD, Gueorguiev B, Alini M, et al.
Artificial intelligence accurately detects traumatic thoracolumbar fractures on sagittal radiographs.Medicina(Kaunas)。2022;58(8):998.
Murata K, Endo K, Aihara T, Suzuki H, Sawaji Y, Matsuoka Y, et al.Artificial intelligence for the detection of vertebral fractures on plain spinal radiography.Sci Rep. 2020;10(1):20031.
CAS一个 Google Scholar一个
Weng YS, Wang LJ, Huang JQ, Cai LJ.Factors associated with new fractures in adjacent vertebrae after percutaneous vertebroplasty for osteoporotic vertebral compression fractures.Am J Transl Res.2024;16(11):6972–9.
Lorentzon M, Litsne H, Axelsson KF.The significance of recent fracture location for imminent risk of hip and vertebral fractures—a nationwide cohort study on older adults in Sweden.Osteoporos int。2024;35(6):1077–87.
Zech JR, Santomartino SM, Yi PH.Artificial intelligence (AI) for fracture diagnosis: an overview of current products and considerations for clinical adoption, from the AJR special series on AI applications.AJR Am J Roentgenol.2022;219(6):869–78.
Oakden-Rayner L, Gale W, Bonham TA, Lungren MP, Carneiro G, Bradley AP, et al.Validation and algorithmic audit of a deep learning system for the detection of proximal femoral fractures in patients in the emergency department: a diagnostic accuracy study.柳叶刀数字健康。2022;4(5):e351–8.
CAS一个 Google Scholar一个
Tadavarthi Y, Vey B, Krupinski E, Prater A, Gichoya J, Safdar N, et al.The state of radiology AI: considerations for purchase decisions and current market offerings.Radiol Artif Intell.2020;2(6):e200004.
Chen M, Cai R, Zhang A, Chi X, Qian J. The diagnostic value of artificial intelligence-assisted imaging for developmental dysplasia of the hip: a systematic review and meta-analysis.J Orthop Surg Res.2024;19(1):522.
Bradley CS, Verma Y, Maddock CL, Wedge JH, Gargan MF, Kelley SP.A comprehensive nonoperative treatment protocol for developmental dysplasia of the hip in infants.Bone Joint J. 2023;105-B(8):935–42.
Liu C, Xie H, Zhang S, Mao Z, Sun J, Zhang Y. Misshapen pelvis landmark detection with local-global feature learning for diagnosing developmental dysplasia of the hip.IEEE Trans Med Imaging.2020;39(12):3944–54.
Huang B, Xia B, Qian J, Zhou X, Zhou X, Liu S, et al.Artificial intelligence-assisted ultrasound diagnosis on infant developmental dysplasia of the hip under constrained computational resources.J Ultrasound Med.2023;42(6):1235–48.
Xu W, Shu L, Gong P, Huang C, Xu J, Zhao J, et al.A deep-learning aided diagnostic system in assessing developmental dysplasia of the hip on pediatric pelvic radiographs.Front Pediatr.2022;9:785480.
Jaremko JL, Hareendranathan A, Bolouri SES, Frey RF, Dulai S, Bailey AL.AI aided workflow for hip dysplasia screening using ultrasound in primary care clinics.Sci Rep. 2023;13(1):9224.
CAS一个 Google Scholar一个
Ghasseminia S, Lim AKS, Concepcion NDP, Kirschner D, Teo YM, Dulai S, et al.Interobserver variability of hip dysplasia indices on sweep ultrasound for novices, experts, and artificial intelligence.J Pediatr Orthop.2022;42(4):e315–23.
Libon J, Ng C, Bailey A, Hareendranathan A, Joseph R, Dulai S. Remote diagnostic imaging using artificial intelligence for diagnosing hip dysplasia in infants: results from a mixed-methods feasibility pilot study.Paediatr Child Health.2023;28(5):285–90.
Kumm J, Roemer FW, Guermazi A, Turkiewicz A, Englund M. Natural history of intrameniscal signal intensity on knee MR images: six years of data from the osteoarthritis initiative.Radiology.2016;278(1):164–71.
Khan M, Evaniew N, Bedi A, Ayeni OR, Bhandari M. Arthroscopic surgery for degenerative tears of the meniscus: a systematic review and meta-analysis.CMAJ.2014;186(14):1057–64.
Bien N, Rajpurkar P, Ball RL, Irvin J, Park A, Jones E, et al.Deep-learning-assisted diagnosis for knee magnetic resonance imaging: development and retrospective validation of MRNet.PLoS Med.2018;15(11):e1002699.
Fritz B, Marbach G, Civardi F, Fucentese SF, Pfirrmann CWA.Deep convolutional neural network-based detection of meniscus tears: comparison with radiologists and surgery as standard of reference.Skeletal Radiol.2020;49(8):1207–17.
Pedoia V, Norman B, Mehany SN, Bucknor MD, Link TM, Majumdar S. 3D convolutional neural networks for detection and severity staging of meniscus and PFJ cartilage morphological degenerative changes in osteoarthritis and anterior cruciate ligament subjects.J Magn Reson成像。2019;49(2):400–10.
Sokal PA, Norris R, Maddox TW, Oldershaw RA.The diagnostic accuracy of clinical tests for anterior cruciate ligament tears are comparable but the Lachman test has been previously overestimated: a systematic review and meta-analysis.Knee Surg Sports Traumatol Arthrosc.2022;30(10):1795.
Nyland J. The ACL Café Menu: individualised treatment of anterior cruciate ligament injuries—from prevention to conservative treatment, repair and reconstruction.Knee Surg Sports Traumatol Arthrosc.2025。
Štajduhar I, Mamula M, Miletić D, Ünal G. Semi-automated detection of anterior cruciate ligament injury from MRI.Comput Methods Programs Biomed.2017;140:151–64.
Richardson ML.MR protocol optimization with deep learning: a proof of concept.Curr Probl Diagn Radiol.2021;50(2):168–74.
Tran A, Lassalle L, Zille P, Guillin R, Pluot E, Adam C, et al.Deep learning to detect anterior cruciate ligament tear on knee MRI: multi-continental external validation.Eur Radiol.2022;32(12):8394–403.
Liu F, Guan B, Zhou Z, Samsonov A, Rosas H, Lian K, et al.Fully automated diagnosis of anterior cruciate ligament tears on knee MR images by using deep learning.Radiol Artif Intell.2019;1(3):180091.
Zeng W, Ismail SA, Pappas E. Detecting the presence of anterior cruciate ligament injury based on gait dynamics disparity and neural networks.Artif Intell Rev. 2020;53(5):3153–76.
Li X, Huang H, Wang J, Yu Y, Ao Y. The analysis of plantar pressure data based on multimodel method in patients with anterior cruciate ligament deficiency during walking.Biomed Res Int.2016;2016(1):7891407.
Wu R, Guo Y, Chen Y, Zhang J. Osteoarthritis burden and inequality from 1990 to 2021: a systematic analysis for the global burden of disease study 2021. Sci Rep. 2025;15(1):8305.
CAS一个 Google Scholar一个
Conrozier T, Brandt K, Piperno M, Mathieu P, Merle-Vincent F, Vignon E. Reproducibility and sensitivity to change of a new method of computer measurement of joint space width in hip osteoarthritis.Performance of three radiographic views obtained at a 3-year interval.Osteoarthr Cartil.2009;17(7):864–70.
CAS一个 Google Scholar一个
Mulford KL, Kaji ES, Grove AF, Saniei S, Girod-Hoffman M, Maradit-Kremers H, et al.A deep learning tool for minimum joint space width calculation on antero-posterior knee radiographs.J Arthroplast.2025。https://doi.org/10.1016/j.arth.2025.01.038。Google Scholar
一个 Ãœreten K, Arslan T, Gültekin KE, Demir AND, Özer HF, Bilgili Y. Detection of hip osteoarthritis by using plain pelvic radiographs with deep learning methods.
Skeletal Radiol.2020;49(9):1369–74.
Tiulpin A, Thevenot J, Rahtu E, Lehenkari P, Saarakkala S. Automatic knee osteoarthritis diagnosis from plain radiographs: a deep learning-based approach.Sci Rep. 2018;8(1):1727.
Norman B, Pedoia V, Noworolski A, Link TM, Majumdar S. Applying densely connected convolutional neural networks for staging osteoarthritis severity from plain radiographs.J Digit Imaging.2019;32(3):471–7.
Pedoia V, Lee J, Norman B, Link TM, Majumdar S. Diagnosing osteoarthritis from T2 maps using deep learning: an analysis of the entire osteoarthritis initiative baseline cohort.Osteoarthr Cartil.2019;27(7):1002–10.
CAS一个 Google Scholar一个
Hashimoto DA, Rosman G, Rus D, Meireles OR.Artificial intelligence in surgery: promises and perils.Ann Surg.2018;268(1):70–6.
Hughes TM, Dossett LA, Hawley ST, Telem DA.Recognizing heuristics and bias in clinical decision-making.Ann Surg.2020;271(5):813–4.
Jacofsky DJ, Allen M. Robotics in arthroplasty: a comprehensive review.J Arthroplast.2016;31(10):2353–63.
McDonnell JM, Ahern DP, Doinn TÓ, Gibbons D, Rodrigues KN, Birch N, et al.Surgeon proficiency in robot-assisted spine surgery.Bone Joint J. 2020;102-B(5):568–72.
Murphy MP, Brown NM.CORR synthesis: when should the orthopaedic surgeon use artificial intelligence, machine learning, and deep learning?.Clin Orthop Relat Res。2021;479(7):1497–505.
Chen X, Li S, Liu X, Wang Y, Ma R, Zhang Y, et al.Acetabular diameter assessment and three-dimensional simulation for acetabular reconstruction in dysplastic hips.J Arthroplast.2023;38(8):1551–8.
Darwood A, Hurst SA, Villatte G, Tatti F, El Daou H, Reilly P, et al.Novel robotic technology for the rapid intraoperative manufacture of patient-specific instrumentation allowing for improved glenoid component accuracy in shoulder arthroplasty: a cadaveric study.J Shoulder Elbow Surg.2022;31(3):561–70.
Gebremeskel M, Shafiq B, Uneri A, Sheth N, Simmerer C, Zbijewski W, et al.Quantification of manipulation forces needed for robot-assisted reduction of the ankle syndesmosis: an initial cadaveric study.Int J Comput Assist Radiol Surg.2022;17(12):2263–7.
Giorgini A, Tarallo L, Novi M, Porcellini G. Computer-assisted surgery in reverse shoulder arthroplasty: early experience.Indian J Orthop.2021;55(4):1003–8.
Kayani B, Konan S, Tahmassebi J, Pietrzak JRT, Haddad FS.Robotic-arm assisted total knee arthroplasty is associated with improved early functional recovery and reduced time to hospital discharge compared with conventional jig-based total knee arthroplasty.Bone Joint J. 2018;100(B(7)):930–7.
Rossi SMP, Sangaletti R, Andriollo L, Matascioli L, Benazzo F. The use of a modern robotic system for the treatment of severe knee deformities.Technol Health Care.2024;32:3737–46.
Sakakibara Y, Teramoto A, Takagi T, Yamakawa S, Shoji H, Okada Y, et al.Effects of the ankle flexion angle during anterior talofibular ligament reconstruction on ankle kinematics, laxity, and in situ forces of the reconstructed graft.Foot Ankle Int.2022;43(5):725–32.
Yang G, Liu D, Zhou G, Wang Q, Zhang X. Robot-assisted anterior cruciate ligament reconstruction based on three-dimensional images.J Orthop Surg Res.2024;19(1):246.
Twomey-Kozak J, Hurley E, Levin J, Anakwenze O, Klifto C. Technological innovations in shoulder replacement: current concepts and the future of robotics in total shoulder arthroplasty.J Shoulder Elbow Surg.2023;32(10):2161–71.
Herzog MM, Kerr ZY, Marshall SW, Wikstrom EA.Epidemiology of ankle sprains and chronic ankle instability.J Athl Train.2019;54(6):603–10.
Peiffer M, Lewis L, Xie K, Guild TT, Ashkani-Esfahani S, Kwon JY.The influence of talar displacement on articular contact mechanics: a 3D finite element analysis study using weightbearing computed tomography.Foot Ankle Int.2024;45(4):393–405.
Gregersen MG, Dalen AF, Skrede AL, Bjelland Ø, Nilsen FA, Molund M. Effects of fibular plate fixation on ankle stability in a Weber B fracture model with partial deltoid ligament sectioning.Foot Ankle Int.2024;45(6):641–7.
Spindler FT, Gaube FP, Böcker W, Polzer H, Baumbach SF.Value of intraoperative 3D imaging on the quality of reduction of the distal tibiofibular joint when using a suture-button system.Foot Ankle Int.2022;44(1):54–61.
Bajorath J, Kearnes S, Walters WP, Meanwell NA, Georg GI, Wang S. Artificial intelligence in drug discovery: into the great wide open.J Med Chem.2020;63(16):8651–2.
CAS一个 Google Scholar一个
Smalley E. AI-powered drug discovery captures pharma interest.Nat Biotechnol.2017;35(7):604–5.
CAS一个 Google Scholar一个
Brown FK, Sherer EC, Johnson SA, Holloway MK, Sherborne BS.The evolution of drug design at Merck Research Laboratories.J Comput Aided Mol Des.2017;31(3):255–66.
CAS一个 Google Scholar一个
Gangwal A, Lavecchia A. Unleashing the power of generative AI in drug discovery.Drug Discov Today.2024;29(6):103992.
CAS一个 Google Scholar一个
Lowe D. AI designs organic syntheses.自然。2018;555(7698):592–3.
CAS一个 Google Scholar一个
Schneider G. Automating drug discovery.Nat Rev Drug Discov.2018;17(2):97–113.
CAS一个 Google Scholar一个
Gligorijević V, Renfrew PD, Kosciolek T, Leman JK, Berenberg D, Vatanen T, et al.Structure-based protein function prediction using graph convolutional networks.纳特社区。2021;12(1): 3168.
Spencer M, Eickholt J, Cheng J. A deep learning network approach to ab initio protein secondary structure prediction.IEEE/ACM Trans Comput Biol Bioinform.2015;12(1):103–12.
CAS一个 Google Scholar一个
Yuan L, Ma Y, Liu Y. Ensemble deep learning models for protein secondary structure prediction using bidirectional temporal convolution and bidirectional long short-term memory.Front Bioeng Biotechnol.2023;11:1051268.
Offensperger F, Tin G, Duran-Frigola M, Hahn E, Dobner S, Ende CWa, et al.Large-scale chemoproteomics expedites ligand discovery and predicts ligand behavior in cells.科学。2024;384(6694):eadk5864.
CAS一个 Google Scholar一个
Wang F, Liu D, Wang H, Luo C, Zheng M, Liu H, et al.Computational screening for active compounds targeting protein sequences: methodology and experimental validation.J Chem Inf Model.2011;51(11):2821–8.
CAS一个 Google Scholar一个
Ren F, Aliper A, Chen J, Zhao H, Rao S, Kuppe C, et al.A small-molecule TNIK inhibitor targets fibrosis in preclinical and clinical models.Nat Biotechnol.2025;43(1):63–75.
CAS一个 Google Scholar一个
Yang X, Wang Y, Byrne R, Schneider G, Yang S. Concepts of artificial intelligence for computer-assisted drug discovery.Chem Rev. 2019;119(18):10520–94.
CAS一个 Google Scholar一个
Averta G, Della Santina C, Valenza G, Bicchi A, Bianchi M. Exploiting upper-limb functional principal components for human-like motion generation of anthropomorphic robots.J Neuroeng Rehabil.2020;17(1):63.
Zhao Y, Liang C, Gu Z, Zheng Y, Wu Q. A new design scheme for intelligent upper limb rehabilitation training robot.Int J Environ Res Public Health.2020;17(8):2948.
Miller-Jackson TM, Natividad RF, Lim DYL, Hernandez-Barraza L, Ambrose JW, Yeow RC-H.A wearable soft robotic exoskeleton for hip flexion rehabilitation.Front Robot AI.2022;9:835237.
Rossi SMP, Panzera RM, Sangaletti R, Andriollo L, Giudice L, Lecci F, et al.Problems and opportunities of a smartphone-based care management platform: application of the Wald principles to a survey-based analysis of patients’ perception in a pilot center.卫生保健。2024;12(2):153.
Chen ZH, Lin L, Wu CF, Li CF, Xu RH, Sun Y. Artificial intelligence for assisting cancer diagnosis and treatment in the era of precision medicine.Cancer Commun (Lond).2021;41(11):1100–15.
Sánchez de la Nava AM, Atienza F, Bermejo J, Fernández-Avilés F. Artificial intelligence for a personalized diagnosis and treatment of atrial fibrillation.Am J Physiol Heart Circ Physiol.2021;320(4):H1337–47.
Ames CP, Smith JS, Pellisé F, Kelly M, Alanay A, AcaroÄŸlu E, et al.Artificial intelligence based hierarchical clustering of patient types and intervention categories in adult spinal deformity surgery: towards a new classification scheme that predicts quality and value.脊柱。2019;44(13):915–26.
Bertsimas D, Masiakos PT, Mylonas KS, Wiberg H. Prediction of cervical spine injury in young pediatric patients: an optimal trees artificial intelligence approach.J Pediatr Surg.2019;54(11):2353–7.
Cattaneo A, Ghidotti A, Catellani F, Fiorentino G, Vitali A, Regazzoni D, et al.Motion acquisition of gait characteristics one week after total hip arthroplasty: a factor analysis.Arch Orthop Trauma Surg.2024;144(5):2347–56.
Johnson WR, Mian A, Lloyd DG, Alderson JA.On-field player workload exposure and knee injury risk monitoring via deep learning.J Biomech.2019;93:185–93.
Pellisé F, Serra-Burriel M, Smith JS, Haddad S, Kelly MP, Vila-Casademunt A, et al.Development and validation of risk stratification models for adult spinal deformity surgery.J Neurosurg Spine.2019;31(4):587–99.
Shohat N, Goswami K, Tan TL, Yayac M, Soriano A, Sousa R, et al.2020 frank stinchfield award: identifying who will fail following irrigation and debridement for prosthetic joint infection.Bone Joint J. 2020;102-B((7 Supple B)):11–9.
Taborri J, Molinaro L, Santospagnuolo A, Vetrano M, Vulpiani MC, Rossi S. A machine-learning approach to measure the anterior cruciate ligament injury risk in female basketball players.Sensors (Basel).2021;21(9):3141.
Tamimi I, Ballesteros J, Lara AP, Tat J, Alaqueel M, Schupbach J, et al.A prediction model for primary anterior cruciate ligament injury using artificial intelligence.Orthop J Sports Med.2021;2021(9):23259671211027544.
Pedoia V, Lansdown DA, Zaid M, McCulloch CE, Souza R, Ma CB, et al.Three-dimensional MRI-based statistical shape model and application to a cohort of knees with acute ACL injury.Osteoarthr Cartil.2015;23(10):1695–703.
CAS一个 Google Scholar一个
Alzubaidi L, Al-Dulaimi K, Salhi A, Alammar Z, Fadhel MA, Albahri AS, et al.Comprehensive review of deep learning in orthopaedics: applications, challenges, trustworthiness, and fusion.Artif Intell Med.2024;155:102935.
Himeur Y, Al-Maadeed S, Kheddar H, Al-Maadeed N, Abualsaud K, Mohamed A, et al.Video surveillance using deep transfer learning and deep domain adaptation: towards better generalization.Eng Appl Artif Intell.2023;119:105698.
Jakubovitz D, Giryes R, Rodrigues MRD.Generalization error in deep learning.In: Boche H, Caire G, Calderbank R, Kutyniok G, Mathar R, Petersen P, editors.Compressed sensing and its applications: third international MATHEON conference 2017. Cham: Springer;2019. p.153–93.
Roy S, Pal D, Meena T. Explainable artificial intelligence to increase transparency for revolutionizing healthcare ecosystem and the road ahead.Netw Model Anal Health Inform Bioinforma.2023;13(1):4.
Arnab A, Miksik O, Torr PHS.On the robustness of semantic segmentation models to adversarial attacks.IEEE Trans Pattern Anal Mach Intell。2020;42(12):3040–53.
Oliveira e Carmo L, van den Merkhof A, Olczak J, Gordon M, Jutte PC, Jaarsma RL, et al.An increasing number of convolutional neural networks for fracture recognition and classification in orthopaedics.Bone Jt Open.2021;2(10):879–85.
George MP, Bixby S. Frequently missed fractures in pediatric trauma: a pictorial review of plain film radiography.Radiol Clin North Am.2019;57(4):843–55.
Allen B, Agarwal S, Coombs L, Wald C, Dreyer K. 2020 ACR data science institute artificial intelligence survey.J am coll radiol。2021;18(8):1153–9.
Roy S, Meena T, Lim SJ.Demystifying supervised learning in healthcare 4.0: a new reality of transforming diagnostic medicine.Diagnostics (Basel).2022;12(10):2549.
Naik N, Hameed BMZ, Shetty DK, Swain D, Shah M, Paul R, et al.Legal and ethical consideration in artificial intelligence in healthcare: who takes responsibility?.Front Surg.2022;9:862322.
Dallora AL, Anderberg P, Kvist O, Mendes E, Diaz Ruiz S, Sanmartin BJ.Bone age assessment with various machine learning techniques: a systematic literature review and meta-analysis.PLOS一个。2019;14(7):e0220242.
CAS一个 Google Scholar一个
致谢
The authors would like to acknowledge the professional language editing provided by Springer Nature Author Services.Additionally, the authors utilized ChatGPT (version GPT-4o, developed by OpenAI) to assist in improving the clarity and readability of the manuscript.
资金
This work was supported by the National Key Research and Development Program of China (2024YFC2510602), the Key Program of the National Natural Science Foundation of China (82230071), the General Program of the National Natural Science Foundation of China (82172098, 82371603), and the Shanghai Hospital Development Center (SHDC2023CRT013).
道德声明
道德批准并同意参加
Not applicable.
Consent for publication
Not applicable.
竞争利益
The authors declare that they have no competing interests.
Rights and permissions
开放访问本文允许以任何媒介或格式的使用,共享,适应,分发和复制允许使用,分享,适应,分发和复制的国际许可,只要您适当地归功于原始作者和来源,就可以提供与创意共享许可证的链接,并指出是否进行了更改。The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material.If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visithttp://creativecommons.org/licenses/by/4.0/。The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
关于这篇文章
引用本文
Song, J., Wang, GC., Wang, SC.等。Artificial intelligence in orthopedics: fundamentals, current applications, and future perspectives.Military Med Res 12, 42 (2025).https://doi.org/10.1186/s40779-025-00633-z
已收到:
公认:
出版:
doi:https://doi.org/10.1186/s40779-025-00633-z