MALDI-TOF MS的血清肽生物标志物以及机器学习,用于诊断和分类肝甲基甲癌
作者:Loilome, Watcharin
介绍
肝胰腺甲状腺菌(HPB)癌症,包括胆管癌(CCA),肝细胞癌(HCC),胆囊癌(GBC)和胰腺导管性腺癌(PDAC),通常是最具侵略性的恶性症,通常是在诊断的最侵略的恶意中。1,,,,2。手术仍然是唯一的治疗治疗方法,但可切除性率取决于诊断时的肿瘤类型和分期3。即使在可能的手术切除,复发率也很高,并且长期生存仍然有限3,,,,4,,,,5,,,,6。因此,早期和准确的诊断对于改善患者预后至关重要。当前的成像技术7例如计算机断层扫描(CT)和磁共振成像(MRI)是HPB癌症诊断的重要工具,但具有局限性,尤其是在早期阶段将恶性与非恶性条件区分开来。此外,这些成像方式需要专门的专业知识来准确解释,这可能并不总是很容易获得。与CT和MRI相关的高成本也可以限制其可访问性,尤其是在资源有限的设置中,进一步强调了对替代诊断方法的需求。组织活检是诊断的黄金标准,是侵入性的,由于肿瘤的位置或患者状况,可能并不总是可行的8。同时,常规的肿瘤生物标志物(例如癌胚抗原(CEA),碳水化合物抗原19(CA19-9)和α-易蛋白蛋白(AFP)被广泛使用,但遭受了较差的特异性和敏感性,尤其是早期疾病。例如,CA19-9通常在胰腺癌和CCA中升高,但缺乏足够的诊断精度,因为在非恶性胆道状况下也可以增加9,,,,10,,,,11,,,,12。同样,AFP是一种用于HCC的常用生物标志物,在检测HCC时具有截止值的局限性13。这些挑战强调了需要新颖,微创和更可靠的生物标志物来改善早期检测和诊断的需求。肽生物标志物已成为癌症诊断的有前途的候选者,反映了肿瘤进展,微环境变化和代谢改变
14,,,,15。肽是由蛋白水解过程产生的小蛋白质片段,可以在血清,血浆和尿液等生物流体中检测到它们14。它们在流通和对特定癌症类型的潜在特异性方面的稳定性使其具有吸引力的诊断工具16,,,,17,,,,18,,,,19。在HPB癌症中,基于肽的生物标志物可以促进早期发现和与非癌状况的分化,最终有助于临床决策。最近的研究表明,肽组在癌症诊断中的潜力,基于质谱的方法鉴定了包括CCA和HCC在内的多种恶性肿瘤中独特的肽特征20,,,,21。特别是,基质辅助激光解吸/电离飞行时间(MALDI-TOF)质谱(MS)基于基于的平台,可以快速肽分析,并已成功地用于区分癌症和非癌症条件22,,,,23,,,,24,,,,25,,,,26。但是,对多种HPB癌症肽生物标志物的全面研究仍未得到探索。
这项研究旨在确定能够使用MALDI-TOF MS将HPB癌和健康个体区分开的候选肽质量指纹(PMFS)。此外,使用机器学习算法来提高分类精度并为HPB癌症诊断开发预测模型。此外,我们评估了测试集中的分类性能,以评估不同样本组的模型的鲁棒性和概括性。这些发现可能有助于开发新型的非侵入性诊断工具,最终改善HPB癌症的检测和分类。
结果
术前实验室发现和肿瘤生物标志物水平
在这项研究中,总共招募了297名参与者,其中包括五组:健康对照(HC;n= 50),胆管癌(CCA;n= 138),胆囊癌(GBC;n= 16),肝细胞癌(HCC;n= 65)和胰腺导管腺癌(PDAC;n= 28)。在进行临床和肽组分析之前,将样品分为训练集(n= 198)和一个测试集(n= 99)。实验结果表明,与健康个体相比,肝胆汁脱脂和胰腺(HPB)癌症患者的肝功能参数(ALT,AST,ALP,胆红素)和肿瘤生物标志物(CEA,CA19-9)显着升高。具体而言,在训练集中,CCA患者的AST和ALT的中位水平分别为32和29 U/L,而健康对照中的AST和ALT水平分别为21和18 U/L。PDAC患者的ALT(72 U/L)和AST(66 U/L)的中位水平最高,有些值达到300 U/L,表明严重的肝受累。类似地,与健康个体(中位数为0.5 mg/dl;范围0.1 -6.4毫克/dl)相比,特别在PDAC患者(中位4.0 mg/dL;范围为0.5 rang 31.0 mg/dl)中,特别是在PDAC患者中观察到胆红素升高的升高。在癌组中,白蛋白水平往往略低,反映出肝脏合成功能受损,尽管差异在统计学上并不显着。就肿瘤标记而言,CEA和CA19-9均显示出较大的变异,并且在癌组中趋于更高。
但是,在各种癌症类型中没有发现这些标记的统计学上显着差异。
例如,CCA中的CA19-9水平中位数为31.13 U/mL,GBC中的27.51 U/mL,HCC中的23.56 U/mL,培训队列中PDAC的25.5 U/mL。CEA水平显示出类似的升高模式,尤其是在CCA和PDAC病例中,但癌症类型之间没有统计学意义。
测试集在训练组中表现出一致的趋势。HPB癌患者,尤其是在PDAC中,肝酶和胆红素水平持续存在,尤其是在PDAC中,PDAC的AST(中位94 U/L)和ALT(中位93 U/L)水平最高。但是,不同癌症类型之间的比较并未揭示这些参数上统计学上的显着差异。总的来说,这些结果表明,虽然肝功能测试和肿瘤生物标志物可以将癌症患者与健康个体区分开,但它们不足以区分HPB恶性肿瘤的特定癌症类型(Table' 1)。
用于肝胰腺癌癌诊断的肽质量指纹
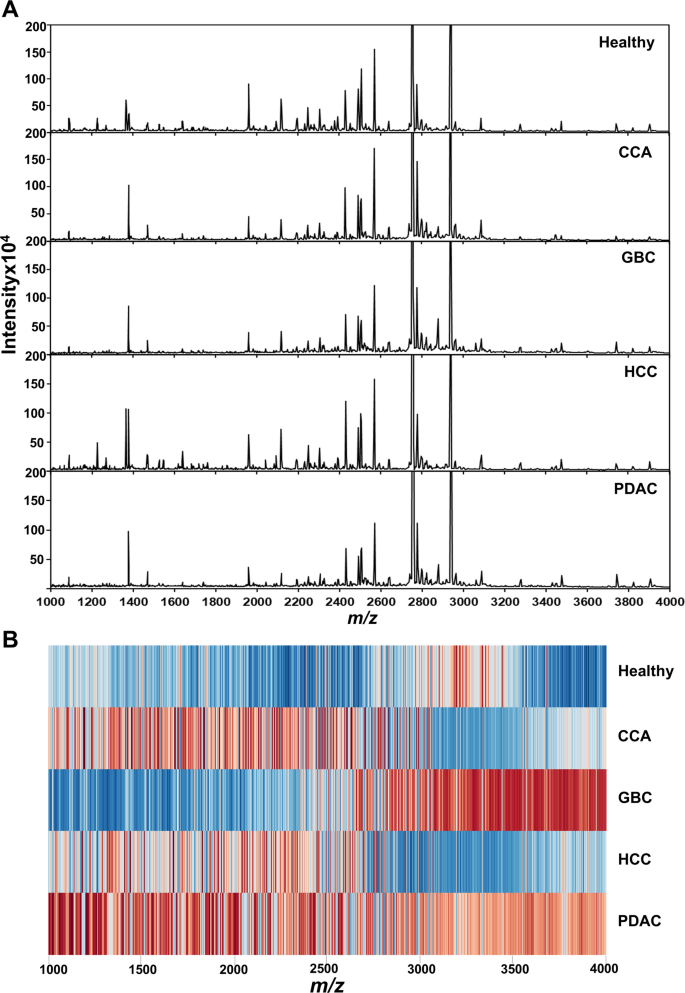
在MALDI-TOF MS设定的训练中,总共检测到1,100个肽特征,该训练的血清表现出明显不同的肽质量指纹(PMF),在健康和HPB癌之间显示(PMFS)(图。 1一个)。将PMF光谱转化为表达z得分,并将其视为热图,以表示健康个体和癌症患者中肽质量指纹表达。总共有1,100个肽m/z分析了1000范围的4000范围。在热图中,红色表示肽表达上调,而蓝色表示每个相应的表达下调m/z位置。结果表明,健康组和HPB癌症之间的PMF表达模式有明显的区别(图。 1b)。
选择用于肝胰腺癌癌分类的关键肽质量指纹
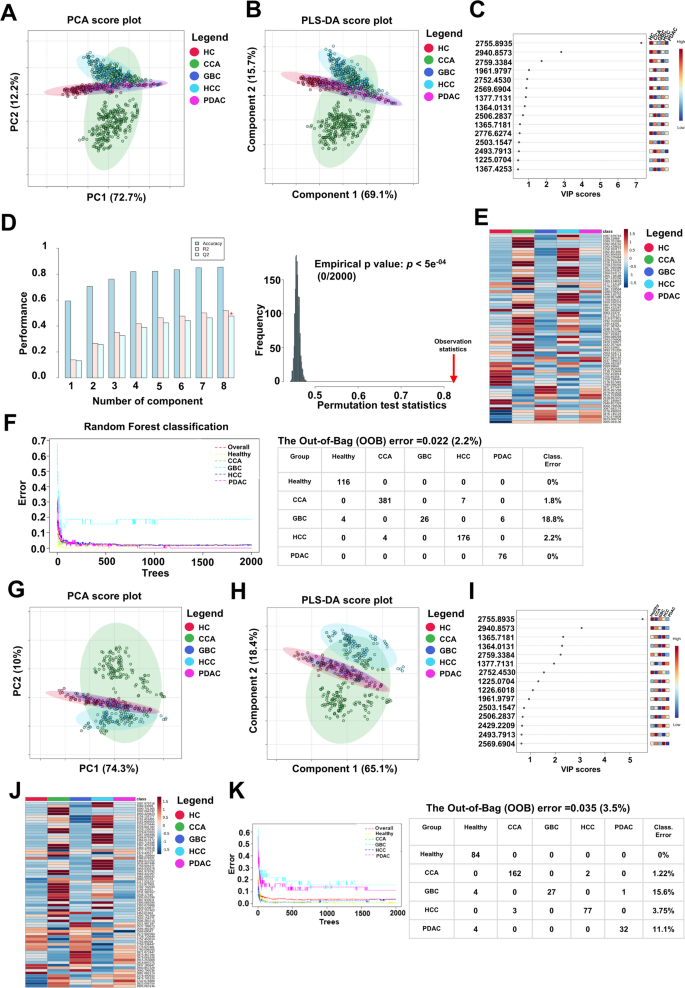
进行了全球PMF分析以识别能够区分健康个体和HPB癌症患者的肽特征。在训练集中,使用Metaboanalyst 6.0分析了从MALDI-TOF MS得出的1,100个肽峰。专门在训练集上进行特征选择,以防止信息泄漏并保持下游分析的完整性。从部分最小二乘判别分析(PLS-DA)和统计显着性(VIP)评分方面的重要性(VIP)评分方面具有变化的肽(VIP)。p从单向方差分析中保留了<0.05)。这导致了71种肽被认为是区分组的信息特征。
在培训集中,主成分分析(PCA)表明,前两个组件占总方差的84.9%(PC1 = 72.7%,PC2 = 12.2%)。如图所示 2A观察到CCA和HCC组之间的部分分离,而健康的对照,GBC和PDAC组倾向于聚集在一起,这表明后者组之间共有的肽表达谱。同样,有监督的分类方法PLS-DA证明了类似的聚类模式。前两个潜在变量解释了84.8%的变化(图 2b),组分布与PCA结果一致。尽管PLS-DA通常由于其监督性质而改善了群体分离,但在这种情况下,组之间的分离与PCA中观察到的分离相似,这表明没有模型监督的情况下,组之间的内在差异已经很明显。通过分析包含1,100肽的全局PMF数据集进一步支持这些发现,这些数据集在PCA和PLS-DA得分图中均表现出一致的分布模式(补充图S1A和B)。VIP评分最高的前15个肽特征被确定为这种分离的关键因素(图 2c)。PLS-DA模型的交叉验证表现出强大的鲁棒性,随着其他组件的增加,râ²和qâ²值的增加。最佳模型达到了Râ²= 0.564和qâ²= 0.502,表明良好的解释性和预测性能。此外,使用2,000次迭代进行置换测试证实没有过度拟合(p<5â-10â»';图 2D)。
基于训练和测试集中71个选定的肽特征的多变量分析和分类性能。((一个)PCA得分图显示了CCA和HCC组的部分分离,在训练集中,健康对照,GBC和PDAC组的聚类聚类。((b)PLS-DA得分图,基于71个选定的肽,证明了与PCA的可比组分布。((c)由PLS-DA模型从VIP分数排名的前15个肽特征。((d)PLS-DA交叉验证结果显示了最佳模型性能(R2= 0.564,q2= 0.502),进行置换测试(n= 2,000)确认训练组中没有过度拟合。((e)跨组平均肽表达水平的热图,肽通过质量增加而排序。((f)随机森林模型分类结果,达到2.2%的脱口而言(OOB)错误率。((g,,,,h)独立测试集中的PCA和PLS-DA得分图,显示了与训练集一致的聚类模式。((我)在测试集中的15个VIP级肽,其中13个与训练集中鉴定的肽重叠。((j)测试集中的平均肽表达水平的热图,显示出一致的表达趋势。((k)测试集中的RF分类性能,产生3.5%的OOB错误率。
热图说明了每组71种肽的表达模式,基于每组平均肽表达的质量增加,显示出不同的差异表达谱(图。 2e)。补充图S2A中也介绍了每个参与者的单个肽表达谱,强调了组内部和组之间的一致变化。
为了进一步评估分类性能,使用71个选定的肽构建了一个随机森林(RF)模型。该模型达到了仅2.2%的脱离(OOB)错误率,当使用所有1,100个肽时观察到的5.56%错误率(补充图S1C)时观察到的5.56%错误率。亚组分类错误率类似:健康对照组和PDAC的0%,CCA为1.8%,HCC为2.2%。唯一值得注意的错误分类发生在GBC组中,错误率为18.8%(图。 2f)。为了评估71个选定肽特征的鲁棒性和概括性,将相同的分析工作流程应用于独立的测试集。
PCA和PLS-DA得分图(图 2gâh)展示了聚类模式,这些模式紧密反映了训练集中观察到的聚类模式。在CCA和HCC组之间保持了部分分离,而健康的对照,GBC和PDAC组继续更加紧密地聚集,这表明表达趋势相似。这些观察结果支持跨独立数据集的基于肽的分类的稳定性。在以VIP分数排名的前15个特征中,有13个与训练集中鉴定的肽重叠,进一步表明了判别标记的强可重现性(图。 2我)。测试集中的交叉验证和置换测试证实了PLS-DA模型的可靠性,没有过度拟合的迹象(补充图S3)。
基于平均表达并通过质量增加的71肽的热图也揭示了群体特异性的表达谱(图。 2j),这与训练集中看到的那些是一致的。在补充图S2B中显示了个体水平的肽表达谱,突出了一致的组内和组间变化。
此外,RF模型在测试集中表现出持续的分类性能,达到3.5%的OOB错误率(图。 2k)。总的来说,这些发现增强了71个选定的肽的歧视能力和可重复性,并强调了它们作为基于肽质量指纹的生物标志物的潜在效用,以区分HPB癌症亚型和健康个体。
使用支持矢量机和随机森林模型的肝甲基甲基甲癌诊断PMF中的分类性能调查
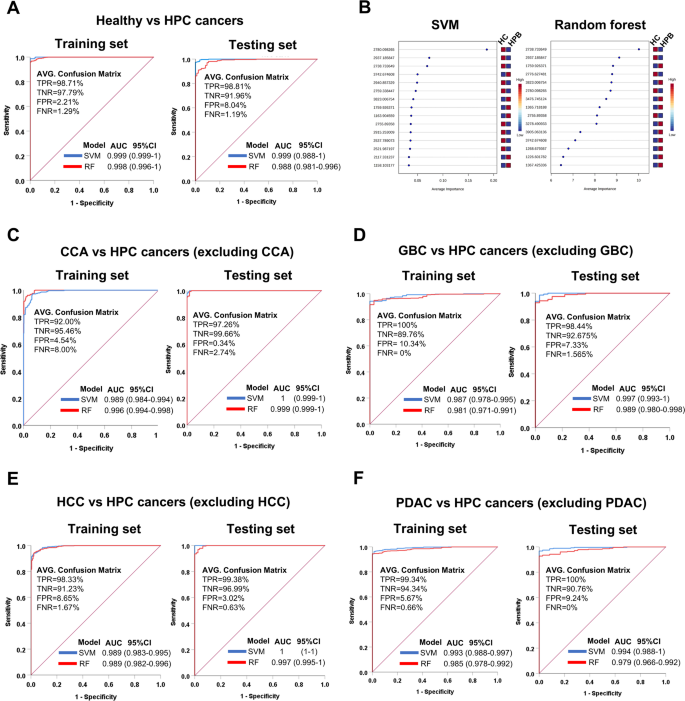
为了评估71个候选PMF的诊断性能,采用了二进制分类模型支持向量机(SVM)和随机森林(RF),以区分健康个体与HPB CANCERS患者,包括CCA,GBC,HCC和PDAC。这些模型是使用带有默认设置的基于Web的Metaboanalyst平台构建的。性能评估是基于混淆矩阵所计算得出的,包括准确性,精度,召回,F1得分以及接收器操作特征曲线(AUC-ROC)下的面积。此外,还计算了Matthews相关系数(MCC),以提供对分类性能的更加平衡的评估。MCC在涉及班级分布不平衡的二进制分类问题中特别有价值,因为它考虑了混淆矩阵的所有四个类别(真正的阳性,真实的负面因素,假阳性和假否定性),并提供了比单独准确性更平衡的措施。MCC值为 +1表示完美的预测,0表示随机预测,1表示预测和观察之间的总分歧。
在训练集中(n198),SVM模型表现出卓越的分类性能,以区分健康个体的精度达到98.74%(n= 29)来自所有癌症病例(n= 169),精度为99.70%,召回98.82%,F1得分为0.99,TNR为98.28%,MCC为0.95,ROC为0.999。与每种癌症类型的个人比较揭示了CCA,GBC和HCC(MCC = 1.00)的完美分类,并且PDAC几乎是完美的分类(MCC =â=â0.99)(表格 2;图 3一个)。在测试集中(n= 99),SVM性能保持稳健。对于所有癌症病例,该模型的精度为98.55%,MCC为0.97,ROC为0.999。健康个体与每种癌症类型之间的比较也显示出很高的MCC值:CCA(0.99),GBC(0.94),HCC(1.00)和PDAC(0.94)(表 2;图 3一个)。
RF模型也表现良好。在训练集中,健康与所有癌症分类的精度为97.10%,精度为99.85%,召回96.75%,MCC为0.90,ROC为0.998。在所有情况下,与个别癌症类型的比较均显示出完美的分类(MCC = 0.99- .00)(表格 3;图 3一个)。在测试集中,RF的健康与所有癌症组的性能略低(准确性:89.14%,MCC:0.76,ROC:0.988)。单个比较的MCC值如下:CCA(1.00),GBC(0.81),HCC(1.00)和PDAC(0.87)(表格) 3;图 3一个)。
此外,为了确定分类最重要的肽,计算了SVM和RF模型的平均重要性度量。在RF中,肽的重要性来自树木的准确性的平均降低,而SVM则使用递归功能消除(RFE)和交叉验证通过其贡献来对特征进行排名。平均重要性得分反映了每个肽在所有模型迭代中的平均影响。两种模型的重要性得分最高的前15个肽均保持一致,表明尽管他们的学习方法不同,但两种算法都确定了一组相似的关键特征(图。 3b)。这种一致性指出了坚固的,独立于模型的特征,从而增强了这些肽在区分样品组时的生物学和统计相关性。跨模型的重要特征的收敛性增强了对其预测价值的信心,表明观察到的分类性能是由强,可重现的信号驱动的,而不是特定于模型的。
此外,我们还利用71个PMF来开发SVM和RF模型,以使用单VS-ALL(OVA)分类策略对HPB癌症进行分类。采用了这种方法来支持潜在的临床应用,因为HPB癌症经常出现重叠的解剖位置,从而使鉴别诊断具有挑战性。因此,在这种情况下,采用了OVA策略来增强模型的判别能力。
在训练集中,SVM模型在将每种HPB癌症亚型与其余癌症类型区分开时表现出卓越的性能。HCC与其他HPB癌的分类精度最高(准确性= 94.97%,McCâ= 0.88,Rocâ= 0.989),其次是PDAC(准确度= 94.67%,MCC = = 0.80,ROC = roc = roc = 0.993),cca(精度mccâ= 0.85,rocâ= 0.989)和gbc(准确性= 90.98%,mccâ=â= 0.56,rocâ= 0.987)(表格) 4;图 3câf)。值得注意的是,尽管召回率相对较低,尤其是GBC(34.41%),但GBC和PDAC的精度和特异性(TNR)值达到100%。
在测试集中,SVM模型在所有癌症亚型中都保持了高歧视能力。该模型的HCC(准确性= 99.68%,MCCâ= 0.99,Rocâ= 1)和CCA(准确性99.04%,MCC = 0.98,Rocâ=â1),随后是GBC(准确性= 94.87%),MCC = 94.87%,MCC = 2.98.87%,MCC = 2.98 =â= i,rocâ= 0.997)和PDAC(准确性= 91.67%,mccâ= 0.73,rocâ= 0.994)(表格) 4;图 3C-F)。这些结果强调了模型的鲁棒性和可靠性,尤其是在将CCA和HCC与其他HPB癌症类型区分开时。
同样,RF模型在大多数比较中也产生了强大的分类性能。在培训集中,PDAC的分类精度最高(95.12%,McCâ= 0.81,Rocâ= 0.985),其次是CCA(94.38%,MCC = 0.89,Rocâ=â= 0.996),HCC(91.42%,MCC- = 91.42%,0.82,0.82,0.82,Rocâ=icocâ=icocâ=icocâ= 998.98 = 989.(90.24%,mccâ= 0.54,rocâ= 0.981)(表格 5;图 3C-F)。在所有亚型中,精度保持较高(98.68%),但GBC的召回率显着降低(32.65%),类似于SVM模型。
在测试集中,RF模型在CCA方面的表现特别出色(准确性= 97.76%,MCC = 0.96,Rocâ= 0.999)和HCC(准确性= 95.51%,MCC = 0.89 = 0.89,ROC = 0.997),以及少于GB的范围= gbc(= gbc)。mccâ= 0.71,rocâ= 0.989)和pDAC(准确性= 91.99%,mcc = 0.73,rocâ= 0.979)(表格) 5;图 3câf)。这些发现证实了PMF在区分HPB癌症亚型中的一致诊断潜力,SVM和RF模型均表现出高可靠性,尽管SVM通常显示出略高的性能,尤其是在处理某些亚型的类别失衡和召回率方面。
为了降低模型的复杂性,选择了每个组的前五位歧视性肽,并用于在OVA框架下使用SVM和RF算法构建分类模型。如补充图S4 s6所示,与使用71个肽的完整模型相比,在训练和测试集中,训练和测试集的分类性能的分类性能显着降低,从而将肽数量降低至5个。如补充表S1所述,在所有比较中观察到的较低的MCC值特别反映了这种下降。
此外,为了评估基于肽的模型的相对诊断性能,我们使用Stard清单中包含的临床生物标志物(ALT,AST,ALP,ALP,Total Bilirubin,CEA,CEA和CA19-9)构建了其他SVM和随机森林模型。具体而言,我们开发了基于(1)仅作为基线的临床生物标志物的模型,以及(2)临床生物标志物和71个肽质量特征(PMF)的组合。仅使用临床生物标志物(补充表S2â)训练的基线模型与单独使用71 PMF的模型相比,表现出较低的性能,这证明了较低的总体指标证明了性能。这一发现表明,基于肽的特征在我们的数据集中具有较高的歧视能力。值得注意的是,将临床生物标志物添加到71 PMF(补充表S4â5)并不能显着改善模型性能,这表明单独选择的肽足够,并且可能已经捕获了常规生物标志物提供的诊断信息。
这些结果表明,PMF在与HPB癌症患者区分开的情况下提供了高歧视能力。在大多数比较中,特别是在SVM模型中,MCC值高的值也证实了这些模型的可靠性和鲁棒性,即使在存在类不平衡的情况下。SVM和RF分类模型均表现出强大的诊断潜力,可以识别HPB癌症,而在大多数情况下,SVM的表现略有效果,包括更有效地处理类失衡的能力。这些发现强调了PMF作为癌症诊断的有前途的生物标志物的价值,对HPB癌症筛查和检测的临床应用提供了高灵敏度和特异性。除了区分健康的个体和HPB癌症患者外,PMF还表现出在单个HPB癌症亚型之间区分区分的分类表现强大,进一步支持其对一般诊断和精确癌症亚型分类的实用性。
讨论
肝胰腺甲状腺菌(HPB)癌症,包括胆管癌(CCA),肝细胞癌(HCC),胆囊癌(GBC)和胰腺导管腺癌(PDAC),与预性不良相关,甚至是预性诊断,甚至是高高的预性诊断,甚至与较晚的疾病有关切除。现有诊断方式的局限性,例如计算机断层扫描(CT),磁共振成像(MRI)和常规的肿瘤生物标志物,突显了迫切需要对新颖,准确和微创诊断工具的迫切需求,尤其是在及时干预可以显着改善临床兴奋的早期阶段。
肽生物标志物在生物样品中可检测到的氨基酸的短链通过表明存在,进展,对治疗的反应和临床预后,在疾病诊断中起着至关重要的作用。常用的生物标志物包括癌胚抗原(CEA)和癌症抗原19(9)(Ca 19 -9)27在癌症护理中,前列腺癌的前列腺特异性抗原(PSA)28和阿尔茨海默氏病中的淀粉样蛋白β肽17。尽管有些可能缺乏特异性,但这些标记对于评估治疗反应和监测疾病复发至关重要。他们捕获分子改变的能力使它们成为早期检测,提高诊断精度和进化个性化医学的宝贵工具。
在这项研究中,我们评估了使用基质辅助激光解吸/电离飞行时间质谱法(MALDI-TOF MS)生成的血清肽质量指纹(PMF)的诊断潜力(PMFS)。基于肽模式分析(图 1a - b),我们的发现表明,这种方法可以可靠地区分健康的个体和患有各种HPB恶性肿瘤的患者。重要的是,高级统计和机器学习技术的整合使能够识别具有高歧视能力和可重复性的71个肽特征的小组(图 2A e)。此外,在特征选择过程中选择的71个肽用于训练分类模型,然后在独立的测试集中进行评估。为了防止过度拟合并确保模型的可靠性,数据集在分析之前将数据集随机分为培训和测试子集。测试集中的分类模型的性能(图 2F–I) showed comparable accuracy to that observed in the training set, highlighting the robustness and generalizability of the candidate PMFs.These results suggest that the 71-peptide signature captures key molecular features associated with HPB malignancies and possesses strong potential for clinical application in HPB cancers diagnosis and classification.The consistent performance across both training and testing datasets underscores the stability of the peptide-based model and supports its utility as a reliable, non-invasive diagnostic tool.
Furthermore, the integration of machine learning models enhances the predictive capability and objectivity of the diagnostic process, potentially reducing inter-observer variability and ensuring consistent interpretation, even in resource-limited settings.Given its relatively low cost, rapid throughput, and minimal sample volume requirements, the MALDI-TOF MS-based serum peptidome presents a feasible diagnostic solution with the potential for broad clinical implementation.In this study, machine learning algorithms, including support vector machine (SVM) and Random Forest (RF), were applied via the user-friendly MetaboAnalyst platform to evaluate the classification performance of 71 selected peptides.The platform enables non-programmers, such as clinicians, to perform advanced analyses through an intuitive web interface.Subsequently, the model performance was assessed using confusing metrics, including accuracy (ACC), precision, recall, F1-score, true negative rate (TNR), area under the receiver operating characteristic curve (ROC), and the Matthews correlation coefficient (MCC).Notably, MCC provided a balanced evaluation, especially in datasets prone to class imbalance29—a challenge often encountered in clinical research.Both the SVM and RF models, developed from the 71-peptide panel, exhibited exceptional classification performance, demonstrating a robust ability to distinguish healthy individuals from patients with HPB malignancies.The models achieved high accuracy in identifying healthy controls and HPB cancers (Tables 2和3;Fig. 3一个)。These strong results across both training and testing sets emphasize the biological relevance, robustness, and diagnostic potential of the selected PMFs biomarkers for reliable disease classification.
Additionally, the study highlights the use of PMFs as input variables for machine learning models in classifying HPB cancers using the one-vs-all (OvA) strategy.This approach, essential given the overlapping anatomical locations and similar clinical presentations of HPB cancers2improves diagnostic precision and supports modular decision-making in clinical practice.The SVM model exhibited strong discriminative power, with the highest accuracy and MCC for HCC vs. others, followed by PDAC, CCA, and GBC.Precision and specificity reached 100% for GBC and PDAC, though the recall for GBC was lower, indicating difficulty in identifying all positive cases.These results were validated in the testing set, where the model performed nearly perfectly for HCC and CCA, with slightly reduced but still robust metrics for GBC and PDAC, highlighting its potential for accurate differential diagnosis of HPB cancers (Table 4;Fig. 3C-F).The RF model also performed well across most subtypes, with similar results to the SVM model, especially for CCA and HCC.The SVM model had a slight edge in handling class imbalance30particularly for GBC, but both models showed strong diagnostic potential, evidenced by high precision and ROC values (Table 5;Fig. 3C-F).The OvA strategy, despite inherent class imbalances30offers a translational advantage by addressing the primary clinical question of whether a specific cancer type is present.While the one-vs-one (OvO) strategy may provide better separation in early-stage analyses, it becomes less feasible as the number of cancer types increases due to computational constraints31。Thus, OvA remains a practical, scalable approach for real-world clinical applications.Despite its advantages, the OvA strategy introduces class imbalance, especially for underrepresented subtypes like GBC.While stratified sampling and class weighting mitigated this issue, the imbalance could still reduce sensitivity for certain classes.Future studies should aim to acquire more balanced datasets and explore advanced sampling techniques like synthetic minority over-sampling (SMOTE) or ensemble methods to address this challenge32。Additionally, integrating clinical variables or multi-omics data could further enhance the classification performance for challenging subtypes.By refining data balance and model design, future research could develop more accurate, clinically reliable tools for classifying HPB cancers and other cancer types.
Our findings are consistent with studies across several types of cancers, which demonstrated the broad application of peptide-based biomarkers in clinical diagnosis20,,,,22,,,,23,,,,24,,,,33,,,,34。In CCA, PMF analysis has demonstrated robust performance in distinguishing cancer patients from healthy controls20。Importantly, this approach can further stratify CCA recurrence subtypes, offering essential prognostic information35。Given that these subtypes frequently correlate with adverse clinical outcomes regardless of disease stage, such discrimination holds significant clinical value.Similarly, in prostate cancer, MALDI-TOF MS-derived PMFs have demonstrated high discriminatory power, enabling precise differentiation between healthy individuals and prostate cancer patients with exceptional sensitivity and specificity24。In the same way, hepatocellular carcinoma, PMF analysis has revealed distinct peptide signatures that reliably distinguish cancer patients from healthy controls, underscoring their clinical utility as robust diagnostic indicators21。In cervical cancer, PMF analysis using MALDI-TOF MS has uncovered distinctive peptide signatures that effectively discriminate between healthy individuals, patients with precancerous lesions, and those at different stages of malignancy.Notably, specific mass-to-charge (m/z) peaks—1466.91, 1898.01, 3159.09, and 4299.40—were identified as robust discriminators, highlighting their potential as diagnostic biomarkers for disease stratification23。In ovarian cancer, peptide-based biomarkers identified through MALDI-TOF MS have proven highly effective in distinguishing cancer patients from healthy individuals, reflecting strong diagnostic performance36。Notably, these biomarkers also differentiated non-malignant cases—such as patients with ovarian cysts—from both healthy subjects and those with ovarian cancer, demonstrating remarkable specificity37。Additionally, they showed outstanding ability to classify different stages of ovarian cancer, with reported sensitivities between 95% and 97% and a specificity of 97%38。These findings highlight the remarkable versatility and diagnostic power of peptide-based biomarkers across multiple cancer types, positioning them as valuable tools for early detection and precise disease classification.
In addition to their role in cancer diagnostics, peptide biomarkers are increasingly being recognized in various medical fields for their potential in early disease detection and monitoring disease progression.Their non-invasive characteristics, rapid screening, and cost-effectiveness make them especially valuable for distinguishing between normal and diseased states, as well as for assessing changes throughout the course of illness.
​ These findings highlight the pivotal role of peptide-based biomarkers, identified through mass spectrometry (MS), in improving the diagnosis and classification of HPB cancers.Building upon this, our study employs an integrated approach that combines high-throughput screening with predictive modeling to improve HPB cancer detection and stratification.​ Firstly, we successfully identified candidate 71 PMFs that not only facilitate the diagnosis of HPB cancers but also differentiate among various HPB cancer types.Secondly, we implemented machine learning algorithms, including SVM and RF models, constructed from these candidate PMFs.These models achieved high classification accuracy, effectively distinguishing between disease stages and types, thereby supporting personalized diagnostics.Thirdly, we validated the performance of the candidate PMFs using testing sets that were separated prior to PMF identification.The diagnostic efficacy observed in the testing sets closely mirrored that of the training sets, demonstrating the robustness of our approach.Collectively, the integration of MALDI-TOF MS and machine learning models forms a synergistic framework that combines rapid screening and advanced classification.This comprehensive approach holds significant promise for improving the detection and diagnosis of HPB cancers.
In clinical practice, MALDI-TOF MS enables the rapid generation of patient-specific peptide profiles from serum samples, facilitating an efficient and personalized diagnostic approach.These profiles are compared against a comprehensive, established peptide biomarker database, allowing for an immediate and accurate match with known cancer-associated signatures.This innovative technique not only aids in the detection of HPB cancers but also supports the differentiation of various HPB cancer types based on their distinct peptide patterns.By integrating MALDI-TOF MS with advanced machine learning algorithms, we can enhance the diagnostic capability, enabling early detection and more precise classification of HPB cancers as shown in summary in supplementary Fig. S7.This method promises to significantly improve clinical outcomes by providing faster, more reliable diagnostics for better patient management.
While the present study demonstrates promising findings, several limitations should be considered when interpreting and applying the results.First, this study was conducted using a single-institution cohort primarily composed of patients from Northeastern Thailand, where cholangiocarcinoma and liver fluke (Opisthorchis viverrini) infection are highly prevalent.As such, the findings may be more generalizable to populations in endemic areas of Southeast Asia, but caution is warranted when extrapolating these results to non-endemic regions or populations with different etiological backgrounds.Second, the number of cases in certain cancer subtypes, such as gallbladder and pancreatic cancers, was relatively small.This limited sample size may reduce the robustness of the machine learning models, increase the risk of overfitting, and affect the reliability of biomarker identification in these groups.Finally, further subgroup analyses, such as comparisons between precancerous lesions and different cancer stages, are necessary to better understand the stage-specific characteristics and potential diagnostic applications of the identified features.
Taken together, these limitations underscore the importance of cautious interpretation and clearly indicate the need for further studies.Specifically, future multicenter investigations involving larger and more diverse populations, as well as independent external validation cohorts, are essential to confirm and strengthen the clinical applicability and generalizability of our findings.In parallel, potential PMFs that showed diagnostic value in this study will be identified using LC-MS/MS.This identification step is a critical bridge toward the development of a robust biomarker panel, which represents the ultimate goal of MS-based peptidome research for clinical application.
In summary, our study showed that PMF via MALDI-TOF MS serves as a rapid and effective screening tool for detecting peptide patterns associated with HPB cancers.By employing machine learning algorithms such as SVM and RF, we achieved high classification accuracy in distinguishing between healthy individuals and patients with various HPB malignancies across both training and testing datasets.The combined application of MALDI-TOF MS and machine learning algorithms not only improves diagnostic accuracy but also holds substantial potential as an adjunct to traditional diagnostic methods.This integrated approach offers a promising alternative for enhancing the early detection and classification of HPB cancers, thereby facilitating more informed clinical decision-making and potentially improving patient outcomes.
材料和方法
道德批准并同意参加
This study was conducted based on the principles of Good Clinical Practice, the Declaration of Helsinki, and national laws and regulations about clinical studies.In addition, informed consent was obtained from all patients.All processes of this study were accepted and approved by the Khon Kaen University Ethics Committee for Human Research under the reference number HE551404 and HE661318.
Population and sample group
In this study, a total of 297 participants were recruited and split into a training set (n = 198) and a testing set (n = 99).Participants were categorized into five groups: healthy controls (n = 50;training set,n = 29;testing set,n = 21), cholangiocarcinoma (CCA;n = 138;training set,n = 97;testing set,n = 41), gallbladder cancer (GBC;n = 16;training set,n = 8;testing set,n = 8), hepatocellular carcinoma (HCC;n = 65;training set,n = 45;testing set,n = 20), and pancreatic ductal adenocarcinoma (PDAC;n = 28;training set,n = 19;testing set,n = 9).To protect patient privacy and ensure confidentiality, all participants were assigned anonymized identifiers.The list of these anonymized patient IDs for the training and testing sets is provided in Supplementary Table S6.Serum samples from the healthy controls were collected from individuals undergoing health screenings at the Srinagarind Hospital Blood Bank, Faculty of Medicine, Khon Kaen University, with approval granted by the Director of Srinagarind Hospital.Serum samples from the Hepato-pancreato-biliary (HPB) cancer groups were sourced from the biobank at the Cholangiocarcinoma Research Institute, Khon Kaen University.Clinical data for the patients were retrospectively collected from medical records at Srinagarind Hospital, Faculty of Medicine, Khon Kaen University, covering patient information from January, 2017, to December, 2021. Prognostic factors were gathered using a retrospective data collection form from the patient medical records, utilizing the ISAN Cohort database at the Cholangiocarcinoma Research Institute, Faculty of Medicine, Khon Kaen 大学。The data collected included age at diagnosis, gender, histological confirmation, tumor size, cancer grade, surgical margins, lymph and cancer staging.
Sample collection and serum preservation
Serum samples were collected from healthy individuals and patients with hepatobiliary diseases prior to surgical treatment.
Blood was drawn via venipuncture into a 5-milliliter clot blood tube.It was ensured that clot formation was complete before centrifugation.The serum was then separated from red blood cells by centrifugation at 3,000–3,500 RPM at 4 °C for 10 min.The serum was carefully aspirated and aliquoted into 1-microliter portions in Eppendorf tubes to prevent repeated thawing of samples.These aliquots were stored at -80 °C in the biobank of the Cholangiocarcinoma Research Institute, Khon Kaen University, until further analysis.Protein quantification was performed using the Lowry assay.
Peptide barcode analysis using MALDI-TOF MS
Each serum sample was analyzed in quadruplicate (i.e., four technical replicates per patient) to ensure reproducibility and reduce technical variability.For each replicate, the serum was mixed with a matrix solution consisting of α-cyano-4-hydroxycinnamic acid (CHCA) in 50% acetonitrile and 0.1% trifluoroacetic acid at a sample-to-matrix ratio of 1:5.The mixture was then spotted onto a MALDI target plate (MTP 384 ground steel, JEOL, Japan), with each sample applied in 30 replicates.After drying at room temperature, the plate was analyzed using the JMS-S3000 SpiralTOF-Plus (JEOL, Japan) in a linear positive mode, targeting peptide barcodes within a mass range of 1,000 to 10,000 Da.Each sample was subjected to 1,500 laser shots.Data acquisition was performed using JEOL msTornado Control version 1.16 (JEOL, Japan), and subsequent processing was carried out with JEOL msTornado Analysis version 1.15 (JEOL, Japan).Spectra were processed using default settings for smoothing, variance stabilization, baseline correction, and peak detection, followed by export in CSV format for further analysis.Mass binning was applied at 1.0 Da intervals across the 1,000–10,000 Da range.Prior to analysis, the instrument was externally calibrated in positive-ion mode using a set of reference peptides with known mass-to-charge ratios (m/z): Angiotensin II (m/z = 1046), P14R (m/z = 1533), human ACTH fragment 18–39 (m/z = 2465), bovine insulin oxidized B chain (m/z = 3465), and bovine insulin (m/z = 5731).Calibration was performed manually using JEOL msTornado Control version 1.16, ensuring mass accuracy within ± 100 ppm.
Support vector machine model
The Support Vector Machine (SVM) model was used to classify and predict potential biomarkers from the candidate metabolites identified in serum samples.The candidate metabolites were selected based on feature selection criteria according to VIP  ≥ 1 from PLS-DA analysis, ANOVA test: FDR-adjustedp < 0.05, which were considered significant for the discriminatory model.Using the MetaboAnalyst 6.0 platform, 71 PMFs from the training set were first normalized to ensure uniform scaling.The SVM model was applied using a Radial Basis Function (RBF) kernel (default kernel in MetaboAnalyst), and cross-validation techniques such as 10-fold cross-validation were used to assess the model generalizability and to avoid overfitting.The default parameters for the SVM model included a cost value of 1 and gamma value of 1/n, where n is the number of features (default settings in MetaboAnalyst).The model performance was evaluated using classification metrics such as accuracy, precision, recall, F1-score, specificity, Matthews correlation coefficient (MCC), and area under the curve (AUC) based on the training set39。The SVM model developed from the training set was then validated using an independent testing set, and model performance was further assessed through the same classification metrics (accuracy, precision, recall, F1-score, specificity, MCC and AUC) derived from the testing set.
Random forest model
The Random Forest (RF) model was employed to perform binary classification between healthy individuals and patients with HPB cancers based on the 71 selected PMFs.The analysis was conducted using the MetaboAnalyst 6.0 web-based platform40。Prior to model construction, data from the training set were normalized to ensure comparability across features.The default settings provided by MetaboAnalyst were used, which include the use of 500 decision trees (ntree = 500) and a default value of mtry (number of variables randomly sampled as candidates at each split) set to the square root of the total number of features.Model performance was evaluated using 10-fold cross-validation to prevent overfitting and to assess the generalizability of the model.Classification metrics reported included accuracy, precision, recall (sensitivity), specificity, F1-score, AUROC, and MCC.After training, the model was validated using an independent testing set, and the same metrics were calculated to assess predictive performance on unseen data.
Model performance estimation
After classification using SVM and RF models, receiver operating characteristic (ROC) curves were generated to evaluate the overall discriminative ability of each model across various classification thresholds.The AUC was calculated to provide a threshold-independent performance metric, offering an aggregate measure of model performance based on the trade-off between the true positive rate (TPR) and the false positive rate (FPR) at different thresholds.These values were derived from components of the confusion matrix—true positives (TP), false positives (FP), true negatives (TN), and false negatives (FN)—calculated at each threshold.
Following ROC analysis, a classification threshold was selected to construct the confusion matrix for each model.The confusion matrix enabled a detailed evaluation of model performance at the chosen threshold by summarizing classification outcomes into TP, FP, TN, and FN categories.Based on these values, several performance metrics were calculated, including accuracy, sensitivity (recall), specificity, precision, F1-score, and MCC.Notably, MCC was included as it provides a more informative and balanced measure for evaluating binary classification performance, particularly when class distributions are imbalanced.MCC considers all four elements of the confusion matrix and ranges from − 1 (completely incorrect classification) to + 1 (perfect classification), with 0 indicating random performance29。
Bioinformatics analysis of peptidome data
Four technical replicates (quadruplicates) were acquired per sample using MALDI-TOF MS, and these spectra were subsequently subjected to comprehensive bioinformatics analysis, including visualization and statistical assessment.Principal Component Analysis (PCA), Partial Least Squares-Discriminant Analysis (PLS-DA), differential analysis (one-way ANOVA), heatmap visualization, and machine learning approaches such as Support Vector Machine and Random Forest models were conducted using MetaboAnalyst version 6.0 (https://www.metaboanalyst.ca/)。A significance threshold ofp < 0.05 was applied, andp-values below this threshold were considered statistically significant.数据可用性
Raw MALDI-TOF MS data supporting the findings of this study are available from the corresponding author upon reasonable request.
参考
Akhoundova, D., Fritsch, R. M. & Hussung, S. J. h.
T. O. H. Precision oncology for hepato-pancreato-biliary (HPB) cancers state of the art and future directions.5, 52–59 (2020).
Kerekes, D. M. et al.Hepatopancreatobiliary malignancies: time to treatment matters.J. Gastrointest.Oncol。 14, 833–848.https://doi.org/10.21037/jgo-22-1067(2023)。
文章一个 PubMed一个 PubMed Central一个 Google Scholar一个
Maspero, M., Sposito, C., Mazzaferro, V., Ercolani, G. & Cucchetti, A. Cure after surgery for hepato-pancreato-biliary cancers: A systematic review.挖。肝病。57, 1–7.https://doi.org/10.1016/j.dld.2024.06.021(2025)。文章
一个 Google Scholar一个 Margonis, G. A. et al.Rates and patterns of recurrence after curative intent resection for gallbladder cancer: a multi-institution analysis from the US Extra-hepatic biliary malignancy consortium.
HPB (Oxford)。18, 872–878.https://doi.org/10.1016/j.hpb.2016.05.016(2016)。文章一个
PubMed一个 Google Scholar一个 Xiang, Y. J. et al.Recurrence hazard rate in patients with hepatocellular carcinoma and bile duct tumor thrombus: a multicenter observational study.
HPB24 , 1703–1710.https://doi.org/10.1016/j.hpb.2022.04.007(2022)。文章
一个 PubMed一个 Google Scholar一个 Evans, D., Hajibandeh, S., Hajibandeh, S. & Athwal, T. HPB SO43 - Meta-analysis of completion pancreatectomy for local recurrence of pancreatic cancer after the index pancreatectomy.br。
J. Surg。111 https://doi.org/10.1093/bjs/znae271.275 (2024)。Jajodia, A., Soyer, P., Barat, M. & Patlas, M. N. Imaging of hepato-pancreato-biliary emergencies in patients with cancer.
Diagn.间隔。成像。105, 47–56.https://doi.org/10.1016/j.diii.2023.11.002(2024)。文章
一个 PubMed一个 Google Scholar一个 Elbanna, K. Y. & Kielar, A. Z. Computed tomography versus magnetic resonance imaging for hepatic lesion characterization/diagnosis.17
, 159–164https://doi.org/10.1002/cld.1089(2021)。Wu, C. H. et al.Comparative radiological pathological study of biliary intraductal tubulopapillary neoplasm and biliary intraductal papillary mucinous neoplasm.
腹部。radiol。(NY)。42, 2460–2469.https://doi.org/10.1007/s00261-017-1167-7(2017)。文章
一个 PubMed一个 Google Scholar一个 Fujii, M., Okamoto, Y. & Shiode, J. A case of cystic intraductal papillary neoplasm of the bile duct with associated adenocarcinoma.Clin.
J. Gastroenterol.13 , 219–224.https://doi.org/10.1007/s12328-019-01040-3(2020)。文章
一个 PubMed一个 Google Scholar一个 Kim, W. J. et al.Clinicopathological features and Long-Term outcomes of intraductal papillary neoplasms of the intrahepatic bile duct.
J. Gastrointest.外科。 20, 1368–1375.https://doi.org/10.1007/s11605-016-3103-5(2016)。
文章一个 MathSciNet一个 PubMed一个 Google Scholar一个
You, Y. et al.Recurrence after resection for intraductal papillary neoplasm of bile duct (IPNB) according to tumor location.J. Gastrointest.外科。 24, 804–812.https://doi.org/10.1007/s11605-019-04235-8(2020)。
文章一个 PubMed一个 Google Scholar一个
Hanif, H. et al.Update on the applications and limitations of alpha-fetoprotein for hepatocellular carcinoma.World J. Gastroenterol. 28, 216–229.https://doi.org/10.3748/wjg.v28.i2.216(2022)。
文章一个 PubMed一个 PubMed Central一个 Google Scholar一个
Zhao, H. et al.Inflammation and tumor progression: signaling pathways and targeted intervention.信号。Transduct.目标。Ther. 6, 263.https://doi.org/10.1038/s41392-021-00658-5(2021)。
文章一个 PubMed一个 PubMed Central一个 Google Scholar一个
Fan, J., Ning, B., Lyon, C. J. & Hu, T. Y. Circulating peptidome and Tumor-Resident proteolysis.酶 42, 1–25.https://doi.org/10.1016/bs.enz.2017.08.001(2017)。
文章一个 PubMed一个 Google Scholar一个
Li, L., Wu, J., Lyon, C. J., Jiang, L. & Hu, T. Y. Clinical peptidomics: advances in instrumentation, analyses, and applications.BME Front. 4, 0019.https://doi.org/10.34133/bmef.0019(2023)。
文章一个 PubMed一个 PubMed Central一个 Google Scholar一个
Ostrowitzki, S. et al.A phase III randomized trial of gantenerumab in prodromal alzheimer’s disease.Alzheimers Res.Ther. 9, 95.https://doi.org/10.1186/s13195-017-0318-y(2017)。
文章一个 PubMed一个 PubMed Central一个 Google Scholar一个
Goetze, J. P. et al.Cardiac natriuretic peptides.纳特。Reviews Cardiol. 17, 698–717.https://doi.org/10.1038/s41569-020-0381-0(2020)。
文章一个 Google Scholar一个
Hilf, N. et al.Actively personalized vaccination trial for newly diagnosed glioblastoma.自然 565, 240–245.https://doi.org/10.1038/s41586-018-0810-y(2019)。
文章一个 广告一个 PubMed一个 Google Scholar一个
Sandanayake, N. S. et al.Identification of potential serum peptide biomarkers of biliary tract cancer using MALDI MS profiling.BMC Clin.Pathol。 14 https://doi.org/10.1186/1472-6890-14-7(2014)。
Park, H. G. et al.MALDI-TOF MS-based total serum protein fingerprinting for liver cancer diagnosis.分析师 144, 2231–2238.https://doi.org/10.1039/c8an02241k(2019)。
文章一个 广告一个 PubMed一个 Google Scholar一个
Kim, S. S. et al.Quantifiable peptide library bridges the gap for proteomics based biomarker discovery and validation on breast cancer.科学。代表。 13, 8991.https://doi.org/10.1038/s41598-023-36159-4(2023)。
文章一个 广告一个 PubMed一个 PubMed Central一个 Google Scholar一个
Rungkamoltip, P., Roytrakul, S. & Navakanitworakul, R. MALDI-TOF MS analysis of serum peptidome patterns in cervical cancer.Biomedicines 11 https://doi.org/10.3390/biomedicines11082327(2023)。
Sun, J. et al.Evaluation of prostate cancer based on MALDI-TOF MS fingerprinting of nanoparticle-treated serum proteins/peptides.塔兰塔 220, 121331.https://doi.org/10.1016/j.talanta.2020.121331(2020)。
文章一个 PubMed一个 Google Scholar一个
Ploypetch, S. et al.Utilizing MALDI-TOF MS and LC-MS/MS to access serum peptidome-based biomarkers in canine oral tumors.科学。代表。 12, 21641.https://doi.org/10.1038/s41598-022-26132-y(2022)。
文章一个 广告一个 PubMed一个 PubMed Central一个 Google Scholar一个
Lee, J. W. et al.Potential of MALDI-TOF-based serum N-glycan analysis for the diagnosis and surveillance of breast cancer.科学。代表。 10, 19136.https://doi.org/10.1038/s41598-020-76195-y(2020)。
文章一个 广告一个 PubMed一个 PubMed Central一个 Google Scholar一个
Kanda, M. et al.The combination of the serum carbohydrate antigen 19 – 9 and carcinoembryonic antigen is a simple and accurate predictor of mortality in pancreatic cancer patients.外科。今天。44, 1692–1701.https://doi.org/10.1007/s00595-013-0752-9(2014)。文章
一个 PubMed一个 Google Scholar一个 Antonarakis, E. S. et al.The natural history of metastatic progression in men with prostate-specific antigen recurrence after radical prostatectomy: long-term follow-up.
109, 32–39https://doi.org/10.1111/j.1464-410X.2011.10422.x(2012年)。Chicco, D. & Jurman, G. The advantages of the Matthews correlation coefficient (MCC) over F1 score and accuracy in binary classification evaluation.
BMC基因。21 , 6.https://doi.org/10.1186/s12864-019-6413-7(2020)。文章
一个 Google Scholar一个 Gao, X. et al.A multiclass classification using one-versus-all approach with the differential partition sampling ensemble.
工程。应用。艺术品。Intell。 97, 104034.https://doi.org/10.1016/j.engappai.2020.104034(2021)。
文章一个 Google Scholar一个
Krebs, R., Bagui, S. S., Mink, D. & Bagui, S. C. Applying Multi-CLASS support vector machines: One-vs.-One vs. One-vs.-All on the UWF-ZeekDataFall22 dataset.13, 3916 (2024).
Yang, Y. & Mirzaei, G. Performance analysis of data resampling on class imbalance and classification techniques on multi-omics data for cancer classification.PLoS One。19, e0293607.https://doi.org/10.1371/journal.pone.0293607(2024)。
文章一个 PubMed一个 PubMed Central一个 Google Scholar一个
Chaiyarit, P., Taweechaisupapong, S., Jaresitthikunchai, J., Phaonakrop, N. & Roytrakul, S. Comparative evaluation of 5-15-kDa salivary proteins from patients with different oral diseases by MALDI-TOF/TOF mass spectrometry.Clin.Oral Investig。19, 729–737.https://doi.org/10.1007/s00784-014-1293-3(2015)。文章
一个 PubMed一个 Google Scholar一个 Yigitbasi, T. et al.An efficient biomarker panel for diagnosis of breast cancer using surface-enhanced laser desorption ionization time-of-flight mass spectrometry.
生物。代表。 8, 269–274.https://doi.org/10.3892/br.2018.1042(2018)。
文章一个 PubMed一个 PubMed Central一个 Google Scholar一个
Thanasukarn, V. et al.Discovery of novel serum peptide biomarkers for cholangiocarcinoma recurrence through MALDI-TOF MS and LC-MS/MS peptidome analysis.科学。代表。 15, 2582.https://doi.org/10.1038/s41598-025-87124-2(2025)。
文章一个 PubMed一个 PubMed Central一个 Google Scholar一个
Swiatly, A. et al.MALDI-TOF-MS analysis in discovery and identification of serum proteomic patterns of ovarian cancer.BMC Cancer。17, 472.https://doi.org/10.1186/s12885-017-3467-2(2017)。
文章一个 PubMed一个 PubMed Central一个 Google Scholar一个
Wu, S. et al.Identification of serum biomarkers for ovarian cancer using MALDI-TOF-MS combined with magnetic beads.int。J. Clin。Oncol。 17, 89–95.https://doi.org/10.1007/s10147-011-0259-6(2012年)。
文章一个 PubMed一个 Google Scholar一个
Pais, R., Grigaite, R., Lacey, J., Jardine, C. & Iles, R. A. Rapid and affordable screening tool for Early-Stage ovarian cancer detection based on MALDI-ToF MS of blood serum.应用。科学。 12, 3030.https://doi.org/10.3390/app12063030(2022)。
文章一个 Google Scholar一个
Cortes, C. & Vapnik, V. Support-vector networks.马赫。学习。 20, 273–297.https://doi.org/10.1007/BF00994018(1995)。
文章一个 Google Scholar一个
Pang, Z. et al.MetaboAnalyst 6.0: towards a unified platform for metabolomics data processing, analysis and interpretation.核酸res。 52, W398–w406.https://doi.org/10.1093/nar/gkae253(2024)。
文章一个 PubMed一个 PubMed Central一个 Google Scholar一个
致谢
All authors are truly thankful for helpful discussions with the late Prof. Narong Khuntikeo at Department of Surgery, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand, Cholangiocarcinoma Research Institute (CARI), Khon Kaen University, Khon Kaen, Thailand and Cholangiocarcinoma Screening and Care Program (CASCAP), Khon Kaen University, Khon Kaen, Thailand.We are also indebted to all members of CASCAP, particularly the cohort members, and researchers at CARI, Faculty of Medicine, Khon Kaen University for collecting and proofing of CCA patient data.We also acknowledge Professor Ross H. Andrews for editing the MS.
资金
This work was Supported by Research Program from Research Department of Khon Kaen University and the National Research Council of Thailand through the Hub of Knowledge Grant to WL.
道德声明
Competing interests
作者没有宣称没有竞争利益。
附加信息
Publisher’s note
关于已发表的地图和机构隶属关系中的管辖权主张,Springer自然仍然是中立的。
补充信息
Below is the link to the electronic supplementary material.
权利和权限
开放访问This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material.您没有根据本许可证的许可来共享本文或部分内容的改编材料。The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material.If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visithttp://creativecommons.org/licenses/by-nc-nd/4.0/。Reprints and permissions
引用本文
Prajumwongs, P., Titapun, A., Thanasukarn, V.
等。Serum peptide biomarkers by MALDI-TOF MS coupled with machine learning for diagnosis and classification of hepato-pancreato-biliary cancers.Sci代表15 , 29169 (2025).https://doi.org/10.1038/s41598-025-14451-9
已收到:
公认:
出版:
doi:https://doi.org/10.1038/s41598-025-14451-9