Advances in artificial intelligence and precision nutrition approaches to improve maternal and child health in low resource settings
作者:Finkelstein, Julia L.
Introduction
Malnutrition is an urgent threat to human health and disproportionately affects women of reproductive age, pregnant and lactating women, and children1,2,3, due to increased physiological demands to support maternal metabolism, transfer to the fetus, and growth and development. Malnutrition—defined to include undernutrition, such as micronutrient deficiencies (“hidden hunger”) and underweight, and overnutrition, including excess adiposity and metabolic diseases—is complex in its etiology and assessment. The double-burden of malnutrition, or the coexistence of both undernutrition and overnutrition—at an individual, household, or population level—adds further complexity to the development of interventions and programs to meet the nutritional needs in these populations4.
In the context of maternal and child health, particularly low-resource settings such as low- and middle-income countries (LMICs), nutritional interventions include micronutrient supplementation, food fortification, and biofortification approaches, with emphasis on prevention or treatment of acute undernutrition or micronutrient deficiencies at the population level5,6,7. For example, micronutrient supplementation and fortification interventions are among the most cost-effective approaches to improve human health and development, particularly in the context of maternal and child health5,8. This is critical as nutrition prior to conception and early in life is a key determinant of health during the first 1000 days of life and beyond9.
Despite major investments, a recent pooled analysis found 1 in 2 women and children still have at least one micronutrient deficiency3, and 1 in 3 pregnant women (500 million) have anemia, a number that has remained unchanged since 200010. At the same time, a type 2 diabetes mellitus (T2DM) crisis has escalated rapidly particularly in LMICs including in South Asia11. Progress on the Sustainable Development Goals, particularly Goal 2: Zero Hunger, has stagnated or regressed12, and progress on reduction in anemia is not on track to meet the World Health Assembly target (i.e., 50% reduction of anemia in women of reproductive age by 2025)13. Comprehensive strategies to address all forms of malnutrition—that involves both preventive and therapeutic strategies requiring population and/or setting-specific considerations—are urgently needed that are tailored (where appropriate and feasible) to the individual context including biology, genomics, and environment for maximal impact on improving maternal and child health.
Value addition of precision nutrition: assessment and prediction
Nutritional assessment—to identify or prioritize those at risk or with greatest need for interventions and monitoring response—is a critical component of any successful intervention/program, at the community or the clinical levels. Accurate assessment of nutritional status, such as anthropometric, biochemical, clinical, and dietary data, in women, infants, and children is necessary to monitor existing programs, identify gaps, and measure efficacy of interventions. Precision nutrition–using individual-level data to predict how a given person may respond to a nutritional intervention, and tailor the intervention (e.g., optimal diet) for that individual as opposed to a one-size-fits-all, population-level approach, is a next step after assessment14. In addition to anthropometric, biochemical, clinical, and dietary data, and socioeconomic, psychosocial and environmental data14, other factors that are now possible to measure on a larger scale include genetic, metabolic, proteomic, or microbiome signatures, which may predict the response to specific nutritional interventions15,16,17. Given how maternal factors (e.g., diet, inflammation, microbiome) appear to dynamically shape child health outcomes via shared biomarkers, vertical microbiota transmission, particularly in the first 1000 days of life, AI modeling approaches that can jointly predict outcomes across the mother-child dyad are critical to successful precision nutrition approaches.
Precision nutrition has shown promise in adults, particularly in research from higher income settings, and this is reflected by recent investments to investigate precision nutrition in even larger cohorts to account for more individual-level heterogeneity. Zeevi (2015) and Korem (2017) and colleagues found that integrating most or all of these variables improved prediction of an adult individual’s glucose response to a particular food18,19. This has set the stage for larger studies such as Nutrition for Precision Health (NPH), part of the by the All of Us study and funded by the National Institutes of Health (NIH)20. NPH will help establish which components of precision nutrition need to be measured in adults across the United States and inform nutritional assessment and interventions to improve human health and address key challenges in precision nutrition. The generalizability of these findings to populations in other settings with different metabolomes, microbiomes, and host genetics is still an open question. Some of the challenges pertain to how to better understand and model the multiple and multi-level risk factors for adverse health outcomes to inform care, prevention, and treatment guidelines. Further challenges include limitations associated with implementing AI methods in precision nutrition in low- and middle-income settings, including incomplete or poor-quality data, technological infrastructure gaps, lack of digital literacy, and ethical and regulatory considerations.
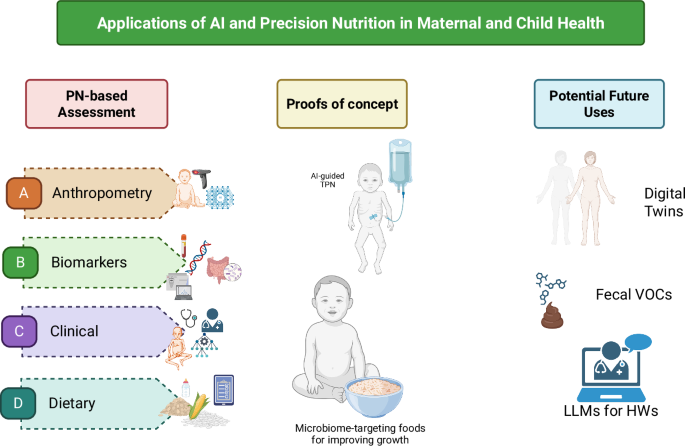
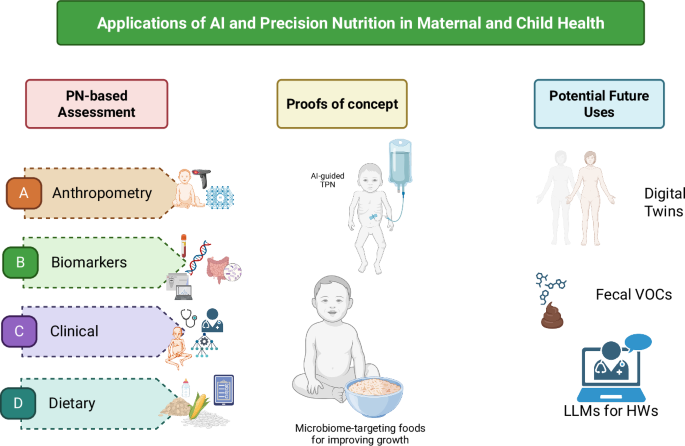
Herein, we discuss the implications of using a precision nutrition lens to improve nutritional assessment with a focus on maternal and child health, particularly in low resource settings. We then outline examples of where a precision nutrition approach is being applied in maternal and child health. Finally, we review the technological landscape supporting precision nutrition to identify potential areas to complement conventional practices, with an emphasis on nutritional assessment, screening, and interventions to improve maternal and child health. These are summarized in Fig. 1.
AI and machine learning (ML) have been used in maternal and child health, particularly image-based assessment methods for (A) Anthropometry and body composition measures and (C) Clinical applications such as predicting intra-uterine growth restriction based on demographic and health characteristics. Past uses of AI and ML to define potential novel (B) Biomarkers for nutrient intakes, such as gut microbial signatures66 have the potential to be applied in maternal and child health, and app-based assessment of (D) Dietary intakes has been successful particularly in estimating most macronutrients among adolescents in a low-resource setting89. Two examples where PN-based methods in maternal and child health have been used include “total parenteral nutrition (TPN) 2.0” for neonates in neonatal intensive care units90 and microbiome-targeting foods in the context of moderate acute malnutrition (MAM)91. Finally, we show potential applications for AI/ML-based approaches in maternal and child health, including digital twins, fecal volatile organic compounds (VOC) for predicting mortality in children with severe acute malnutrition (SAM)96, and the use of large language model (LLM)-powered coaching for frontline health workers’ (HW) training on early childhood development curricula102. Figure created in BioRender (Huey S, Sinha S, Mehta S (2025) https://BioRender.com/fn7t1bw) and finalized via All Time Design.
Assessment of nutritional status
Nutritional assessment includes anthropometric measures and body composition, biochemical markers, clinical signs and symptoms, and dietary intake. These are measured and analyzed through direct measures of the body, blood collection and analysis, clinical exams, and interviews with trained personnel. Such methods have been used for decades in nutrition research and have resulted in major gains in the evidence base for nutrition and maternal and child health. However, these methods for assessment are time-, personnel- and resource-intensive, and depend on the availability of trained staff and equipment to collect and process the data, which can range from relatively simple (e.g., body weight data in a small study) to large and complex (e.g., 24-h recall data and food composition databases from repeated samples in a large cohort of mother-infant dyads)21. These methods may be complemented by integrating novel approaches to leverage increased computational power and efficiency to analyze complex multi-modal data, via artificial intelligence (AI) and machine learning (ML) models16,22. AI involves using a computer to model intelligent behavior with little human guidance23. ML is a mathematical tool that facilitates the development of algorithms to make accurate predictions from large datasets with greater accuracy than traditional statistical methods, and is of increasing interest to nutrition and health research22,24,25. Incorporating novel AI and ML methods to nutrition assessment could enable faster, more efficient, and more accurate data, translating to more accurate models and findings and inform the development and monitoring of nutritional interventions, including for maternal and child health.
Anthropometry and body composition
Established methods for nutritional assessment, including anthropometry and body composition, using measurement tapes, length/height boards, and skinfold calipers, are time-intensive and challenging to perform; require trained personnel (and may have high level of inter- and intra-rater variability in measurements); and do not automatically store data digitally, necessitating an additional data entry step26,27,28. For body composition assessment, gold standard methods include dual-energy X-ray absorptiometry (DXA), densitometry, or air displacement plethysmography (bioelectric impedance analysis (BIA),reviewed in refs. 29,30 may not be suitable for pregnant women or children), and require equipment that is costly to purchase and maintain controlled conditions, and may be less feasible in field or resource-limited settings31.
As optical imaging devices have become relatively inexpensive over the past few decades, interest in digital or automated measures of anthropometry and body composition has increased27. Three-dimensional scanning devices can objectively and relatively quickly measure the body, process the acquired data, and calculate circumferences, volumes, lengths, and surface areas27, although estimation of body composition measures (e.g., fat mass, fat free mass) from this data is more challenging32. A 3-dimensional (3D) scanner can complete hundreds of anthropometry measures in seconds32, though 3D imaging scanners vary in portability33. Smartphone and mobile phone-based technologies have advanced such that automated optical scanning systems33 and BIA34,35,36 may be captured via mobile phone, in addition to 2D images taken by the phone’s camera. These portable methods could be developed and validated for data collection in field settings at the point of need30.
Machine learning approaches are well-suited to process data from images from 3D scanners or camera-enabled mobile devices to estimate anthropometry and body composition given that (a) image data analysis can be automated reducing personnel time required; and (b) the algorithm “learns” or becomes trained and accuracy is increased as additional curated data are ingested37. In addition, taking photos of participants is faster and less labor-intensive than anthropometry or body composition measurements, with contactless data collection. For example, ML methods have been used to predict body height from single-depth images in adults by researchers such as Lokshin and colleagues38 and in multiple studies39,40. In children, height measurements and predictions can be used to detect stunting; a 2021 study in India found that a convolutional neural network-based method accurately predicted the height of standing children under 5 years of age within an acceptable 1.4 cm range among 70% of depth images, which were generated from videos from captured on a commercial 2016 smartphone; however, details on the degree of inaccuracy of the remaining 30% of depth images was not reported41. However, image and video quality are key for accurate modeling; indeed, videos with noisy data (e.g., blurry, dark, lack of the subject, or too many participants) were identified and removed from the test datasets41. In adults (n = 12 females and n = 15 males) with obesity, a 2022 study found that an automated machine learning method analyzing data from smartphone camera-enabled capture and analysis of 2D images was able to reproducibly and accurately estimate whole body fat mass compared to DXA (correlation R2 = 0.99)24, with no differences by sex. However, estimating body composition in children using image-based machine learning techniques and validating such tools in the field in pregnant women and young children remain research gaps.
Deep learning algorithms can help process images and videos but require secure server availability for app-based intake estimation for sustainability. In the future, use of convolutional neural networks or other architectures such as generative adversarial networks and deep learning algorithms will be key to process the large image-based datasets. The increased computing power helps to identify more minute details in the pictures and in the process improves accuracy42. Current methods for anthropometry and body composition assessment are constrained by high throughput. Advancing the technology to enhance reliability and reproducibility, and to optimize for individuals across the life cycle in the form of a mobile phone are important for monitoring changes in individual anthropometry or body composition over time in resource-limited settings, to develop and evaluate nutritional interventions and programs.
Biochemical
Nutritional biomarkers such as those measured in blood or urine to quantify dietary intake or nutrition status are objective and less prone to bias due to recall or reporting43. For example, minerals and vitamins can be measured in blood, stable isotopes of doubly labeled water and urine samples can enable measurement of daily total energy expenditure, and 24-h urinary nitrogen can estimate protein intake44,45. One of the main challenges in assessing nutritional status is the limited range of biomarkers that reflect intake and predict functional or clinical outcomes, such as the response to a given dietary intervention in a population. Biomarkers of nutrients and associated metabolites often reflect recent intake rather than sustained dietary habits; adding to this complexity, the metabolic rate for energy and different nutrients has been shown to have inter-individual variation46,47, possibly due to the gut microbiome18,48. In addition, some biomarkers may not accurately reflect status for nutrients that are tightly regulated, such as serum calcium or zinc49,50, in addition to changes in status prompted by inflammation (described below); finally, a limited range of nutritional biomarkers predict functional outcomes or health outcomes.
The interplay of inflammation and nutritional status may influence intra- and inter-individual variation51. The acute phase response involves the release of inflammatory cytokines such TNF-alpha, IL-1, and IL-6, which stimulate the liver to produce acute phase proteins (APP). The APPs include over 200 plasma proteins, an estimated 50% of which are involved in regulation of nutrient transport or status52. For example, serum/plasma ferritin (stored iron), retinol (vitamin A status), and zinc (zinc status) are directly affected by inflammation, both acute and chronic. In the context of acute inflammation, serum/plasma ferritin concentrations increase, whereas retinol and zinc decrease52,53. Iron trafficking may be impacted54, limiting the distribution of iron from blood to cells throughout the body in order to limit its availability to pathogens55; the liver halts the release of retinol and its binding protein49; and the transfer of zinc from blood to liver may increase50. As a result, assessment of these micronutrients without accounting for inflammation (e.g., C-reactive protein (CRP), α−1-acid glycoprotein (AGP) or other inflammatory cytokines56) may result in altered (higher or lower) micronutrient status57.
Several methods are available for population-level adjustment of ferritin and retinol, including Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA); however, not all micronutrients or populations are covered; BRINDA adjustment methods are available for or validated in preschool children, school-aged children, or women of reproductive age (ferritin only), but not in pregnant women or infants53,58,59,60,61. These methods use CRP and/or AGP to adjust micronutrient status to a more accurate concentration without the presence of inflammation. However, these are population-based methods for inflammation adjustment and do not typically apply to the individual level and in the context of illness. Considering that both acute and chronic inflammation (e.g., obesity and metabolic diseases) appear to impact micronutrient concentrations, accounting for inflammation as part of the comprehensive set of variables is important for precision nutrition-based strategies. These biomarkers related to metabolism (metabolites) are part of nutrient metabolism. Metabolomics, the study of these metabolites or unique fingerprints as a result of metabolic processes is an upcoming theme in nutrition research. Recently, ML methodologies such as neural networks were used to prepare an evaluation chart using nutritional biomarkers and tried to link dietary intake with biochemical profile to understand the effect on body weight62. A further challenge is to capture intra- and inter-individual variation in the metabolic and phenotypic response to a dietary intervention and ultimately predict those most likely to respond to a particular type of intervention.
Assessment of biological specimens requires central laboratories, specialized equipment, ensuring cold chain, extensive benchmarking and validation of preservation methods, and trained personnel. Methods and devices that are field-friendly (i.e., portable and not impacted by variation in environment such as temperature and humidity) and that adhere to the ASSURED criteria (i.e., Affordable, Sensitive, Specific, User-friendly, Rapid and robust, Equipment-free, Delivered)63 are particularly relevant in the context of maternal and child health and in lower-resource settings. Additionally, the availability of noninvasive methods or tests that require only small volumes of blood—particularly appropriate for populations like young children and pregnant women—is paramount to successful assessment and monitoring. Biomarker assessment at the point of care has considerable applications for screening and precision nutrition in the context of maternal and child health, particularly in resource-limited settings. For example, point-of-care assessment of vitamin A status in blood has been demonstrated, using chemical reaction that can be miniaturized in a device or test kit to facilitate use in field and community healthcare settings. Methods to screen for vitamin A status using point-of-care methods have been cataloged previously and include portable fluorometers, photometers, immunoassays, and microfluidics-based though only some were commercially available64. These devices have the potential to routinely screen for vitamin A deficiency—and evaluate interventions—particularly in settings with limited resources and infrastructure.
Gut microbiome diversity, composition, and function are also potential novel biomarkers of dietary intake, dietary quality65, nutrition status, and/or response to interventions, including analysis of fecal microbial biomarkers of food intake using AI methods66. These methods need to be implemented and validated in the context of maternal and child health, including during pregnancy, mother-infant dyads, and in children. Diversity in maternal and child populations including ethnicity, habitual diets, socioeconomic status, and age is needed for these types of studies as well as other studies such as those using gut microbiome to predict glycemic response to interventions. Validation across large, diverse populations and over time, as well as repeated analyses among similar cohorts, is needed to ensure reproducibility. In addition, evaluation of novel biomarkers compared to currently used biomarkers. For these biomarkers to accurately reflect dietary intake, further detail is needed regarding food and nutrient composition, since vitamin and mineral content in vegetables varies considerably67 depending on conditions such as season68 or post-harvest processing and storage69. Standardized approaches for biomarker validation, comprehensive and accessible food composition databases, a common ontology for dietary biomarkers, and advances in statistical procedures for novel biomarkers of dietary intake are also needed45.
Clinical
Clinical outcomes include adverse pregnancy outcomes, and metabolic diseases, including cardiovascular disease (CVD), T2DM, metabolic dysfunction-associated steatotic liver disease (MASLD, formerly known as non-alcoholic fatty liver disease (NAFLD)), obesity, hyperlipidemia, and cancers70,71. The physiological changes that occur during pregnancy and the postpartum period may unmask metabolic risk factors such as hypertension and altered glucose metabolism not observed prior to pregnancy, highlighting a key window to use AI methods to monitor risk factors and future cardiovascular outcomes72. In children and adolescents, the prevalence of obesity continues to rise, particularly in low- and middle-income settings, and is linked to persistence of obesity into adulthood and associated comorbidities and premature mortality73.
The applications of ML and AI methods in clinical examination may enable earlier intervention or treatment, particularly for nutrition-related metabolic and non-communicable diseases. Many metabolic diseases and sequelae may be assessed via medical imaging techniques, which are particularly suitable to ML and AI methods72,74,75. For example, AI-based processing and assessment of retinal images enabled early detection of retinopathy related to T2DM76. Training ML models on MRI-derived images of liver fat content along with other ‘omics and clinical data have also been used to diagnose NAFLD and outperformed existing prediction tools77. Other AI methods such as convolutional neural networks can model raw electrocardiogram signatures to detect heart rhythm dysfunctions72. They may also be useful for detecting pregnancy outcomes such as congenital anomalies and intrauterine growth retardation (IUGR)78,79.
The most accurate method for measuring low-density lipoprotein (LDL) requires beta-quantification, which is time-intensive, expensive, and infrequently used. The Friedewald equation was developed to estimate LDL using total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and triglycerides (TG): LDL-C = TC – HDL – TGs/580. However, this equation relies on the assumptions that have not been validated in pregnancy, in children, or in the context of certain health conditions, such as HIV80. Five ML methods—linear regression, random forest, gradient boosting, support vector machine (SVM), and neural network—were used to estimate LDL-C in women with HIV (n = 5,219) or without HIV (n = 2127) compared to the Friedewald equation80. In this study, an SVM algorithm outperformed the other four ML methods and the Friedewald equation. Initial findings from this study offers support for further investigation of ML methods in predicting risk factors for metabolic health outcomes.
In a study using a Bayesian kernel machine regression (BKMR) ML approach, sex-specific differences were observed when 12 dietary components were examined in association with 10-year risk of atherosclerotic CVD81. BKMR was used to incorporate non-linear and interactive associations of dietary components with health outcomes, and account for the high degree of collinearity often observed with dietary intakes. When all other dietary components were held fixed, unprocessed red meat was associated with increased risk for atherosclerotic CVD in women. In men, fruit was non-linearly associated with lower risk atherosclerotic CVD, with an interaction between fruit consumption and whole grains was reported. BKMR identified complexities with multiple dietary intakes in association with CVD and indicate its potential in identifying which nutrient(s) or their interaction(s) are associated with disease risk by sex. Together, these methods enable more targeted precision nutrition approaches or interventions to be developed.
The potential clinical applications for machine learning in the context of precision nutrition and non-communicable diseases is evident, particularly to automate and standardize analysis of medical images. The applications of ML methods to identify risk factors or health outcomes may vary in the clinical context. For example, while there is at least one FDA-approved AI-based device available for diabetic retinopathy screening in adults, which analyzes retinal images using a cloud-based software program to output a positive or negative result82, other clinical outcomes (e.g., CVD and MASLD) are in the development stage and require validation and refinement, Further development and validation of AI and ML methods is needed for early detection of non-communicable diseases to inform early intervention and treatment.
Dietary records
Dietary intake has been identified as a modifiable determinant of individual nutritional status. Current methods for estimating dietary intake include 24-h dietary recall, food frequency questionnaire, multiple-day food records or diet records83. Although self-reported dietary intake has been evaluated using these methods in numerous epidemiological studies, measurement error, day-to-day variability, and intensive training, resources, and time burden for personnel and participants are important limitations. Food composition databases for estimating the macro- and micro-nutrient content and intake of, for example, a 24-h recall, may be limited. For example, in one study, half of the available ~100 food composition databases globally were last updated more than 10 years ago with some only available for 1980-1989, limiting the data on data such as ultra-processed foods84.
It is important to develop and validate methods for accurate dietary intake in pregnancy and during critical periods such as preconception, pregnancy, and early in life. Methods such as wearable devices85,86,87, image-based assessment88, and novel biomarkers66 may be used to accurately capture dietary intakes. For example, ML methods has been used to determine a gut microbial signature of specific whole foods (e.g., broccoli, nuts, barley) in men and women44,66. However, these methods may require additional expertise and time in processing and analyzing the resulting data88. In order to address the gap of dietary intake of adolescent females in low- and middle-income settings, a mobile AI-technology–assisted dietary assessment technique, the “Food Recognition Assistance and Nudging Insights” (FRANI) app was developed and validated against weighed food records as a ground truth in Vietnam89. Dietary intake was assessed on three non-consecutive days (i.e., 2 weekdays, 1 weekend). A smartphone with the FRANI app was provided and participants (12–18 y) were trained to take photos of each instance of food consumption. Equivalence between FRANI and weighed records was determined at the 10% bound for calories, protein, fat, and four micronutrients, and at the 15% and 20% bound for carbohydrate and several other vitamins and minerals, suggesting an accurate estimation of most intakes. Some wider bounds were observed for vitamins A and B12, possibly due to lower frequency of consumption, estimation errors, and large variance, small sample size, and limitations of FRANI in assessing vitamin A-rich fruit and vegetables and vitamin-B12–rich foods in mixed dishes. Other limitations included the need for training, recall bias, changes in eating patterns due to taking photos while eating, and limitations of recognizing less common foods. However, findings demonstrate the potential for AI-assisted dietary intake estimation in the context of maternal and child health, particularly in low-resource settings.
Precision nutrition applied in maternal and child health
In this section, we describe some examples of using precision nutrition approaches to optimize an intervention in a study in infants and children. Advanced AI-based precision nutrition approaches are beginning to be applied in maternal and child health contexts. Engagement of key stakeholders, including scientists, local and public authorities, and healthcare professionals, and incorporation of cultural practices, religion, language, caregiving norms, and family structure into precision nutrition-based recommendations is critical to the success and scale-up of precision nutrition in maternal and child health.
AI-guided precision total parenteral nutrition
Recently, AI methods were used to guide and optimize total parenteral nutrition (TPN) formulas for infants in the neonatal intensive care unit (NICU)90. Using information collected routinely in electronic medical records, the AI model “TPN 2.0” identified 15 specific formulas that improved safety, reduced cost, was rated higher by physicians compared to the current practice in a blinded study, and had fewer morbidities such as necrotizing enterocolitis. This model, employed using transformer architecture, may be scaled to LMICs given that the data for the model are already collected as part of the standard of care.
Microbiota-directed complementary food
Microbiota-directed complementary food (MDCF) has demonstrated success over traditional ready-to-use therapeutic food (RUTF), highlighting the utility of a precision nutrition approach using host gut microbiome as an input variable91,92. RUTFs were designed to treat SAM in children; tailoring for gut microbial composition may be an important additional therapeutic target. Intervention with MDCF twice daily for 3 months increased the abundances of plasma proteins associated with improved growth, bone health, immune function and neurodevelopment in malnourished children in Bangladesh, compared to standard RUTF. Clinically, the mean rate of growth per week was greater in the MDCF group (WLZ; MDCF: 0.021 (0.014, 0.029) vs. RUTF: 0.010 (0.003, 0.017), and specific gut bacteria correlated with WLZ were increased. This intervention was given for only 3 months; longer follow-up studies will determine if this improved growth is sustained over time. Findings are consistent with recent studies in adults that have demonstrated that outcomes of dietary interventions depend on the baseline gut microbiome of the host, which varies by individual18,19. Tailoring diets with microbiome-targeting or directed foods for addressing nutritional and health challenges in children and adults is warranted.
Potential for precision nutrition in maternal and child health
Digital twins
Digital twins are virtual systems (or replicas of machines) used to simulate how a product may be optimized by adjusting one or multiple factors—in a software environment93,94. Originating in engineering, and similar to the counterfactual in epidemiology, the concept of digital twins is increasingly being applied to medicine (e.g., “patient-specific digital twins”). Patient data, collected from the whole body to the subcellular level, can be collected and ingested into devices or algorithms, which calculate for example, the amount of insulin to deliver from an implanted glucose sensor, or risk assessment for thrombosis from clinical measurements93. The concept of “digital twins” is a promising approach that involves integrating biological data from the whole-body to the subcellular level with clinical data from patients. Findings can be used for precision nutrition and medicine to tailor and individualize treatment. The integration of AI for precision nutrition, to predict individual response to a given intervention holds promise, particularly in extending the reach of traditional health care systems in maternal and child health and in low-resource settings. Integration of digital twins into existing healthcare systems needs to address computational complexity, and to tailor nutrition interventions to pregnant women and their children.
Use of fecal volatile organic compounds as a biomarker of gut microbiota composition
Microbiome signatures could be a novel bioindicator to predict response to nutritional interventions. Fecal volatile organic compounds may predict intestinal microbiota composition and metabolic function, given that these compounds are produced in the gut primarily by intestinal microbes as part of the fermentation of non-starch polysaccharides95. Volatile organic compounds (VOCs) can be measured using a commercial portable, self-contained unit96. For example, a recent study in Malawi and Kenya demonstrated that these compounds predicted mortality among hospitalized children with severe acute malnutrition96. In this study, a pipeline of machine learning classifiers were used to compare fecal volatile organic compounds of the children, finding that these profiles were distinct between children who survived and those who died (area under the curve = 0.71), likely reflecting differences in the gut microbiota, although sequencing was not conducted to confirm. The SVM algorithm best predicted child mortality in this study. However, prediction via VOCs is challenged by variability in VOC composition across individuals, standardization issues, and practical application constraints. Further research is needed to inform assessment of VOCs as a biomarker in nutrition studies, to determine or describe normal profiles of VOCs and validate the biomarker in different populations.
Use of plasma proteomics for assessment of anemia and micronutrient deficiencies
The etiology of anemia and micronutrient deficiencies are complex and multifactorial. Hence, a holistic approach is needed to for screening and interventions for prevention and treatment. Validated biomarker panels for micronutrient status assessment is needed to inform screening and interventions in the context of maternal and child health and in low-income settings. In this context, identification and quantification of plasma proteomics97 could help support screening quantification of protein biomarkers of micronutrient status in undernourished children in low- and middle-income settings98. These approaches require minimal sample size and are well-poised to screen for multiple micronutrient deficiencies and inflammatory biomarkers at a time in a single platform99. Determination of the precise cluster of plasma proteins is needed to precisely assess micronutrient and inflammation status, which may be possible via machine learning, and to develop a field-friendly affordable biomarker panel for the assessment at the population level.
Use of deep learning and artificial intelligence for disease diagnosis
Small bowel enteropathies (e.g., Environmental Enteric Dysfunction (EED), celiac disease, tropical sprue, and HIV enteropathy) account for a significant proportion of undernutrition in children in low-income settings. Deep learning and AI-based approaches can be used for screening and diagnosis of small intestinal enteropathies. Histopathology is the gold standard for the diagnosis of all these conditions, but with significant overlaps such as villous blunting and crypt elongation. The application of convolutional neural networks for image analysis demonstrated accuracy in the diagnosis of EED and celiac disease100. The use of deep learning methods on the images obtained by video capsule endoscopy is another example of AI-based application for disease detection101. However, more precise tools or approaches are needed to diagnose enteropathies to mitigate the nutrition-related burden and consequences.
LLM-powered coach training frontline health workers on early childhood development curricula
In South Africa, LLMs, a form of artificial neural network based on generative AI, are being developed to support frontline health care workers102. These LLMs will distill information and content on early childhood development and the Kangaroo Mother Care method, promoting skin-to-skin contact and self-care for exclusive breastfeeding, particularly for infants born preterm or low birth weight103. The curriculums developed by the LLMs will be tested for safety, accuracy, usability, and added value. This is one of 50 innovations recognized by the Bill & Melinda Gates Foundation Grand Challenges Initiative to harness LLMs to reduce global inequality.
Limitations and challenges of using AI in maternal and child health
There are several limitations and challenges to use of AI in the context of maternal and child health and in resource-limited settings. Methodological limitations include model generalizability, data privacy, and potential biases in the training data. National and regional guidelines are needed for regulatory frameworks for data privacy. Population-specific training datasets are needed for development and validation of algorithms for maternal and child health and in LMICs. Application and feasibility limitations include fitting in AI-based assessments or predictions into already overworked, under-resourced healthcare staff. Identification of key variables that explain inter- and intra-individual variation is needed to inform precision nutrition interventions.
Conclusions
Malnutrition continues to represent a major threat to maternal and child health, particularly in low- and middle-income settings. Precision nutrition methods need to be integrated into screening and interventions for maternal and child health and in LMICs. Further research is needed to establish the benefits of precision nutrition in maternal and child health, particularly in LMICs, which may lack the infrastructure and resources to implement precision nutrition into routine practice, as well as have very different microbiome compositions and diets. In resource-constrained settings, limited clinical, laboratory, and financial resources may constrain routine nutritional assessment and microbiome and phenotyping assessments. While technological advances are driving the cost of these innovations down, recent advances in laboratory and quantitative methods and technologies can enhance accessibility at the point of care. Further, precision nutrition approaches need to socio-cultural contexst, preferences, and the food environment. Privacy concerns will need to be addressed, particularly considering the novel methods with optical imaging and body composition. With computational power widely accessible and increasingly more affordable around the world, an AI and ML approaches may democratize the practice of public health and medicine far more rapidly than other methods. Precision nutrition—and integration of AI and ML methods and improved computational power and technological advancements—needs to be integrated to nutritional screening, diagnosis, and treatment, and inform the development of nutritional interventions to improve maternal and child health. Precision nutrition approaches can help enhance the design, monitory, and evaluation of interventions to improve maternal and child health.
References
WHO. Malnutrition. In Fact Sheets (ed World Health Organization) (WHO, 2021).
UNICEF, WHO, International Bank for Reconstruction and Development/The World Bank. Levels and trends in child malnutrition: UNICEF/WHO/World Bank Group Joint Child Malnutrition Estimates: key findings of the 2023 edition. In Joint Child Malnutrition Estimates 2023 Edition (UNICEF and WHO, 2023).
Stevens, G. A., Beal, T., Mbuya, M. N. N., Luo, H. & Neufeld, L. M. Global Micronutrient Deficiencies Research Group. Micronutrient deficiencies among preschool-aged children and women of reproductive age worldwide: a pooled analysis of individual-level data from population-representative surveys. Lancet Glob. Health 10, e1590–e1599 (2022).
Wells, J. C. et al. The double burden of malnutrition: aetiological pathways and consequences for health. Lancet 395, 75–88 (2020).
Keats, E. C. et al. Effective interventions to address maternal and child malnutrition: an update of the evidence. Lancet Child Adolesc. Health 5, 367–384 (2021).
Larsen, B., Hoddinott, J. & Razvi, S. Investing in nutrition: A global best investment case. J. Benefit Cost Anal. 14, 235–254 (2023).
Bhutta, Z. A. et al. What works? Interventions for maternal and child undernutrition and survival. Lancet 371, 417–440 (2008).
Victora, C. G. et al. Revisiting maternal and child undernutrition in low-income and middle-income countries: variable progress towards an unfinished agenda. Lancet 397, 1388–1399 (2021).
Bhutta, Z. A. Early nutrition and adult outcomes: pieces of the puzzle. Lancet 382, 486–487 (2013).
WHO. Daily Iron Supplementation in Adult Women and Adolescent Girls, Vol 33. (World Health Organization, 2016).
Ali, S. H., Bhattacharya, S., Chanda, A. & Dhar, B. South Asia’s diabetes crisis needs families: how can we advance from informal care to integrated engagement? Lancet Reg. Health Southeast Asia 38, 100607 (2025).
UN. The Sustainable Development Goals Report Special Edition (ed United Nations) (United Nations, 2023).
WHO. Global Nutrition Targets 2025: Anaemia Policy Brief (WHO/NMH/NHD/14.4) (World Health Organization, 2014).
NIH. 2020-2030 Strategic Plan for NIH Nutrition Research: A Report of the NIH Nutrition Research Task Force (ed National Institutes of Health) (National Institutes of Health, 2020).
Mehta, N. H. et al. Potential mechanisms of precision nutrition-based interventions for managing obesity. Adv. Nutr. 15, 100186 (2024).
Kirk, D., Catal, C. & Tekinerdogan, B. Precision nutrition: a systematic literature review. Comput Biol. Med. 133, 104365 (2021).
Huey, S. L. et al. Precision nutrition-based interventions for the management of obesity in children and adolescents up to the age of 19 years. Cochrane Database Syst. Rev. 1, CD015877 (2025).
Korem, T. et al. Bread affects clinical parameters and induces gut microbiome-associated personal glycemic responses. Cell Metab. 25, 1243–1253 e1245 (2017).
Zeevi, D. et al. Personalized nutrition by prediction of glycemic responses. Cell 163, 1079–1094 (2015).
NIH. Nutrition for Precision Health, powered by the All of Us Research Program. In Common Fund Programs (ed NIH) (NIH, 2023).
Bedsaul-Fryer, J. R. et al. Precision nutrition opportunities to help mitigate nutrition and health challenges in low- and middle-income countries: an expert opinion survey. Nutrients 15, 3247 (2023).
Lee, B. Y. et al. Research gaps and opportunities in precision nutrition: an NIH workshop report. Am. J. Clin. Nutr. 116, 1877–1900 (2022).
Hamet, P. & Tremblay, J. Artificial intelligence in medicine. Metabolism 69S, S36–S40 (2017).
Farina, G. L., Orlandi, C., Lukaski, H. & Nescolarde, L. Digital single-image smartphone assessment of total body fat and abdominal fat using machine learning. Sensors 22, 8365 (2022).
Rativa, D., Fernandes, B. J. T. & Roque, A. Height and weight estimation from anthropometric measurements using machine learning regressions. IEEE J. Transl. Eng. Health Med. 6, 4400209 (2018).
Mocini, E. et al. Digital anthropometry: a systematic review on precision, reliability and accuracy of most popular existing technologies. Nutrients 15, 302 (2023).
Heymsfield, S. B. et al. Digital anthropometry: a critical review. Eur. J. Clin. Nutr. 72, 680–687 (2018).
Conkle, J. & Martorell, R. Perspective: are we ready to measure child nutritional status with lasers?. Adv. Nutr. 10, S10–S16 (2019).
Willett, W. Anthropometric Measures and Body Composition (Oxford University Press, 2013).
Kuriyan, R. Body composition techniques. Indian J. Med. Res. 148, 648–658 (2018).
Pepper, M. R. et al. Validation of a 3-dimensional laser body scanner for assessment of waist and hip circumference. J. Am. Coll. Nutr. 29, 179–188 (2010).
Pleuss, J. D. et al. A machine learning approach relating 3D body scans to body composition in humans. Eur. J. Clin. Nutr. 73, 200–208 (2019).
Bourgeois, B. et al. Clinically applicable optical imaging technology for body size and shape analysis: comparison of systems differing in design. Eur. J. Clin. Nutr. 71, 1329–1335 (2017).
Choi, A. et al. Smartphone-based bioelectrical impedance analysis devices for daily obesity management. Sensors 15, 22151–22166 (2015).
Villa, F. et al. Wearable multi-frequency and multi-segment bioelectrical impedance spectroscopy for unobtrusively tracking body fluid shifts during physical activity in real-field applications: a preliminary study. Sensors 16, 673 (2016).
Heymsfield, S. B. et al. Mobile evaluation of human energy balance and weight control: potential for future developments. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2015, 8201–8204 (2015).
Teno, J. M. Garbage in, garbage out-words of caution on big data and machine learning in medical practice. JAMA Health Forum 4, e230397 (2023).
Lokshin, M. M., Sajaia, Z. & Azari, S. Survey specialists and data scientists meet: machine learning to measure a person’s height from a picture. In WorldBank Blogs (2018).
Pleuss JD et al. A machine learning approach relating 3D body scans to body composition in humans. Eur. J. Clin. Nutr. 73, 200–208 (2019).
Lee, D-s., Kim, J-s., Jeong, S. C. & Kwon, S-k. Human height estimation by color deep learning and depth 3D conversion. Appl. Sci. 10, 5531 (2020).
Trivedi, A. et al. Height estimation of children under five years using depth images. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2021, 3886–3889 (2021).
Eppenhof, K. A. J., Lafarge, M. W., Veta, M. & Pluim, J. P. W. Progressively trained convolutional neural networks for deformable image registration. IEEE Trans. Med. Imaging 39, 1594–1604 (2020).
Shim, J. S., Oh, K. & Kim, H. C. Dietary assessment methods in epidemiologic studies. Epidemiol. Health 36, e2014009 (2014).
Frankenfeld, C. L. Fecal bacteria as an addition to the lineup of objective dietary biomarkers. J. Nutr. 151, 273–274 (2021).
Maruvada, P. et al. Perspective: dietary biomarkers of intake and exposure-exploration with omics approaches. Adv. Nutr. 11, 200–215 (2020).
Lampe, J. W. & Chang, J. L. Interindividual differences in phytochemical metabolism and disposition. Semin. Cancer Biol. 17, 347–353 (2007).
Miles-Chan, J. L. & Harper, M. E. Deconstructing interindividual variability in energy metabolism: implications for metabolic health. Am. J. Physiol. Endocrinol. Metab. 325, E107–E112 (2023).
Jardon, K. M., Canfora, E. E., Goossens, G. H. & Blaak, E. E. Dietary macronutrients and the gut microbiome: a precision nutrition approach to improve cardiometabolic health. Gut 71, 1214–1226 (2022).
Tanumihardjo, S. A. et al. Biomarkers of Nutrition for Development (BOND)—vitamin A review. J. Nutr. 146, 1816S–1848S (2016).
King, J. C. et al. Biomarkers of Nutrition for Development (BOND)—zinc review. J. Nutr. 146, 858S–885S (2016).
Thurnham, D. I. Interactions between nutrition and immune function: using inflammation biomarkers to interpret micronutrient status. Proc. Nutr. Soc. 73, 1–8 (2014).
Raiten, D. J. et al. Inflammation and nutritional science for programs/policies and interpretation of research evidence (INSPIRE). J. Nutr. 145, 1039S–1108S (2015).
Thurnham, D. I., Northrop-Clewes, C. A. & Knowles, J. The use of adjustment factors to address the impact of inflammation on vitamin A and iron status in humans. J. Nutr. 145, 1137S–1143S (2015).
Suchdev, P. S. et al. Assessment of iron status in settings of inflammation: challenges and potential approaches. Am. J. Clin. Nutr. 106, 1626S–1633S (2017).
Lynch, S. et al. Biomarkers of Nutrition for Development (BOND)—iron review. J. Nutr. 148, 1001S–1067S (2018).
Colt, S. et al. Vitamin A status, inflammation adjustment, and immunologic response in the context of acute febrile illness: a pilot cohort study among pediatric patients. Clin. Nutr. 40, 2837–2844 (2021).
Thurnham, D. I. et al. Using plasma acute-phase protein concentrations to interpret nutritional biomarkers in apparently healthy HIV-1-seropositive Kenyan adults. Br. J. Nutr. 100, 174–182 (2008).
Suchdev, P. S. et al. Overview of the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Adv. Nutr. 7, 349–356 (2016).
Thurnham, D. I. et al. Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: a meta-analysis. Am. J. Clin. Nutr. 92, 546–555 (2010).
Barffour, M. A. et al. Comparability of inflammation-adjusted vitamin A deficiency estimates and variance in retinol explained by C-reactive protein and alpha1-acid glycoprotein during low and high malaria transmission seasons in rural Zambian children. Am. J. Trop. Med. Hyg. 98, 334–343 (2018).
Barffour, M. A. et al. Malaria exacerbates inflammation-associated elevation in ferritin and soluble transferrin receptor with only modest effects on iron deficiency and iron deficiency anaemia among rural Zambian children. Trop. Med. Int. Health 23, 53–62 (2018).
Panagoulias, D., Sotiropoulos, D. & Tsihrintzis, G. Nutritional biomarkers and machine learning for personalized nutrition applications and health optimization. In 12th International Conference on Information, Intelligence, Systems & Applications (IISA)) (2021).
Kettler H., White K. & Hawkes S. Mapping the Landscape of Diagnostics for Sexually Transmitted Infections (ed Bank/WHO UUW) (UNICEF/UNDP/World Bank/WHO, 2004).
Huey, S. L. et al. A review of portable quantitative and semi-quantitative devices for measurement of vitamin A in biological samples. Curr. Res. Biotechnol. 4, 253–274 (2022).
Maskarinec, G. et al. Fecal microbial diversity and structure are associated with diet quality in the multiethnic cohort adiposity phenotype study. J. Nutr. 149, 1575–1584 (2019).
Shinn, L. M. et al. Fecal bacteria as biomarkers for predicting food intake in healthy adults. J. Nutr. 151, 423–433 (2021).
Davidson, J. & LeClerc, J. A. The variation in the mineral content of vegetables. J. Nutr. 11, 55–66 (1936).
Phillips, K. M. et al. Seasonal variability of the vitamin C content of fresh fruits and vegetables in a local retail market. J. Sci. Food Agric. 98, 4191–4204 (2018).
Bouzari, A., Holstege, D. & Barrett, D. M. Vitamin retention in eight fruits and vegetables: a comparison of refrigerated and frozen storage. J. Agric. Food Chem. 63, 957–962 (2015).
Chew, N. W. S. et al. The global burden of metabolic disease: data from 2000 to 2019. Cell Metab. 35, 414–428.e413 (2023).
Brown, H. L. et al. Promoting risk identification and reduction of cardiovascular disease in women through collaboration with obstetricians and gynecologists: a presidential advisory from the American Heart Association and the American College of Obstetricians and Gynecologists. Circulation 137, e843–e852 (2018).
Adedinsewo, D. A. et al. Cardiovascular disease screening in women: leveraging artificial intelligence and digital tools. Circ. Res. 130, 673–690 (2022).
Jebeile, H., Kelly, A. S., O’Malley, G. & Baur, L. A. Obesity in children and adolescents: epidemiology, causes, assessment, and management. Lancet Diabetes Endocrinol. 10, 351–365 (2022).
Sidey-Gibbons, J. A. M. & Sidey-Gibbons, C. J. Machine learning in medicine: a practical introduction. BMC Med. Res. Methodol. 19, 64 (2019).
Martin-Isla, C. et al. Image-based cardiac diagnosis with machine learning: a review. Front Cardiovasc Med. 7, 1 (2020).
Ellahham, S. Artificial intelligence: the future for diabetes care. Am. J. Med. 133, 895–900 (2020).
Atabaki-Pasdar, N. et al. Predicting and elucidating the etiology of fatty liver disease: a machine learning modeling and validation study in the IMI DIRECT cohorts. PLoS Med. 17, e1003149 (2020).
Bekele, W. T. Machine learning algorithms for predicting low birth weight in Ethiopia. BMC Med. Inf. Decis. Mak. 22, 232 (2022).
Deval, R., Saxena, P., Pradhan, D., Mishra, A. K. & Jain, A. K. A machine learning-based intrauterine growth restriction (IUGR) prediction model for newborns. Indian J. Pediatr. 89, 1140–1143 (2022).
Dong, T. et al. Machine learning estimation of low-density lipoprotein cholesterol in women with and without HIV. J. Acquir. Immune Defic. Syndr. 89, 318–323 (2022).
Zhao, Y., Naumova, E. N., Bobb, J. F., Claus Henn, B. & Singh, G. M. Joint associations of multiple dietary components with cardiovascular disease risk: a machine-learning approach. Am. J. Epidemiol. 190, 1353–1365 (2021).
Stark, A. FDA permits marketing of artificial intelligence-based device to detect certain diabetes-related eye problems. In Press Announcements (Food and Drug Administration, 2018).
Baranowski, T. 24-Hour Recall and Diet Record Methods (Oxford University Press, 2013).
de Quadros, V. P. et al. Global trends in the availability of dietary data in low and middle-income countries. Nutrients 14, 2987 (2022).
Alshurafa, N. et al. Association of number of bites and eating speed with energy intake: wearable technology results under free-living conditions. Appetite 167, 105653 (2021).
Kyritsis, K., Diou, C. & Delopoulos, A. Modeling wrist micromovements to measure in-meal eating behavior from inertial sensor data. IEEE J. Biomed. Health Inf. 23, 2325–2334 (2019).
Uehara, F. et al. Impact of masticatory behaviors measured with wearable device on metabolic syndrome: cross-sectional study. JMIR Mhealth Uhealth 10, e30789 (2022).
Bulungu, A. L. S. et al. Validation of a life-logging wearable camera method and the 24-h diet recall method for assessing maternal and child dietary diversity. Br. J. Nutr. 125, 1299–1309 (2021).
Nguyen, P. H. et al. Relative validity of a mobile AI-technology-assisted dietary assessment in adolescent females in Vietnam. Am. J. Clin. Nutr. 116, 992–1001 (2022).
Phongpreecha, T. et al. AI-guided precision parenteral nutrition for neonatal intensive care units. Nat. Med. 31, 1882–1894 (2025).
Chen, R. Y. et al. A microbiota-directed food intervention for undernourished children. N. Engl. J. Med. 384, 1517–1528 (2021).
Gehrig, J. L. et al. Effects of microbiota-directed foods in gnotobiotic animals and undernourished children. Sci. (Wash. D. C.) 365, 139 (2019).
Laubenbacher, R., Sluka, J. P. & Glazier, J. A. Using digital twins in viral infection. Science 371, 1105–1106 (2021).
Tao, F. & Qi, Q. Make more digital twins. Nature 573, 490–491 (2019).
Buijck, M. et al. Sniffing out paediatric gastrointestinal diseases: the potential of volatile organic compounds as biomarkers for disease. J. Pediatr. Gastroenterol. Nutr. 63, 585–591 (2016).
van den Brink, D. A. et al. Prediction of mortality in severe acute malnutrition in hospitalized children by faecal volatile organic compound analysis: proof of concept. Sci. Rep. 10, 18785 (2020).
Lee, S. E., Schulze, K. & West, K. P. Jr. Rainer Gross award lecture 2018: the childhood plasma proteome: discovering its applications in public health nutrition. Food Nutr. Bull. 40, 144–150 (2019).
Cole, R. et al. The plasma proteome identifies expected and novel proteins correlated with micronutrient status in undernourished Nepalese children. J. Nutr. 143, 1540–1548 (2013).
Lee, S. E. et al. Plasma proteome biomarkers of inflammation in school aged children in Nepal. PLoS ONE 10, e0144279 (2015).
Syed, S. et al. Artificial intelligence-based analytics for diagnosis of small bowel enteropathies and black box feature detection. J. Pediatr. Gastroenterol. Nutr. 72, 833–841 (2021).
Adewole S et al. Deep learning methods for anatomical landmark detection in video capsule endoscopy images. In Proceedings of the Future Technologies Conference 2020 Oct 31 (pp. 426-434). (Springer International Publishing, 2020).
Bill & Melinda Gates Foundation. Closing the supervision gap: a large language model (LLM)-powered coach for frontline workers. In Awards (Bill & Melinda Gates Foundation, 2024).
Freeman, M. AI is helping solve malnutrition | Opinion. In Newsweek (2024).
Acknowledgements
Nutrition for Precision Health, powered by the All of Us Research Program is supported by the National Institutes of Health (NIH) Common Fund, which is managed by the Office of the Director/Office of Strategic Coordination (OSC). Consortium components include awards U54TR004279, AOD22022001 (AI Centers), UG1HD107688, UG1HD107691, UG1HD107692, UG1HD107696, UG1HD107697, UG1HD107711 (Clinical Centers), U24CA268153 (Metabolomics and Clinical Assays Center), U24DK131617 (Microbiome and Metagenomics Center), U24CA268228 (Dietary Assessment Center), U24HD107676 (Research Coordinating Center), U24OD023121 (Biobank), U2COD231196, OT2OD035404 (Data and Research Center), and OT2OD030043 (Participant Technology Systems Center). SLH was supported by the National Institutes of Health under award 5 T32 HD087137. The views expressed are those of the authors and do not necessarily reflect those of the NIH or the Department of Health and Human Services of the United States.
Ethics declarations
Competing interests
R.K. is a scientific advisory board member, and consultant for BiomeSense, Inc., has equity and receives income. He is a scientific advisory board member and has equity in GenCirq. He has equity in and acts as a consultant for Cybele. He is a co-founder of Biota, Inc., and has equity. He is a cofounder of Micronoma and has equity and is a scientific advisory board member. He is a board member of Microbiota Vault, Inc. He is a board member of N = 1 IBS advisory board and receives income. He is a Senior Visiting Fellow of HKUST Jockey Club Institute for Advanced Study. The terms of these arrangements have been reviewed and approved by the University of California, San Diego in accordance with its conflict of interest policies. All other authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Anagnostis Argiriou, Konstantinos Rouskas, and the other, anonymous, reviewer for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mehta, S., Huey, S.L., Fahim, S.M. et al. Advances in artificial intelligence and precision nutrition approaches to improve maternal and child health in low resource settings. Nat Commun 16, 7673 (2025). https://doi.org/10.1038/s41467-025-62985-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-62985-3